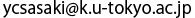World’s First Discovery of Intramolecular Motions of Chaperonin That Plays an Important Role in Protein Repair (Press Release)
- Release Date
- 30 May, 2013
- BL40XU (High Flux)
The University of Tokyo
Tokyo University of Agriculture and Technology
Japan Synchrotron Radiation Research Institute (JASRI)
Key points of study
♦ Key research findings
The research group succeeded in real-time and high-precision monitoring of intramolecular motions of the chaperonin protein at the single-molecule level, which plays an important role in the repair of nonnative proteins.
♦ Novelty of research
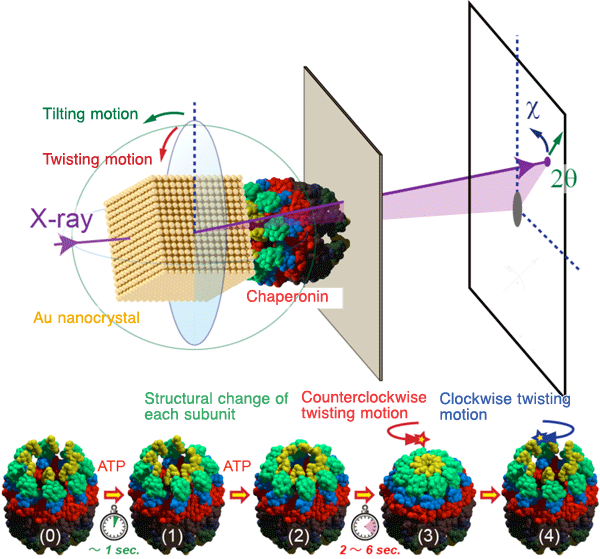
Chaperonin has a cylinder structure in which two oligomeric rings are stacked. After chaperonin binds to adenosine triphosphate (ATP), part of the rings changes; the initially open and built-in lid closes along with a synchronized twisting of the entire ring similarly to the domino effect.
♦ Social significance/future prospect
In molecular biology, molecules are considered as being stationary with a specific structure. High-speed and high-precision monitoring of conformational changes of protein molecule observed in this study may change the strategies for drug development and the concept of intermolecular interaction in the future.
|
A research group led by Yuji Sasaki (professor) of the Graduate School of Frontier Sciences of the University of Tokyo, Hiroshi Sekiguchi (project research associate of the Sasaki Laboratory at that time) of JASRI, and Masafumi Yohda (professor) of the Tokyo University of Agriculture and Technology succeeded in the real-time and high-precision monitoring of intramolecular motions of the chaperonin molecule at the single-molecule level for the first time in the world. Chaperonin plays an important role in the repair of nonnative proteins and has a cylinder structure in which two origomeric rings are stacked. After chaperonin binds to ATP, part of the rings changes; the initially open built-in lid closes along with a synchronized counterclockwise twisting of the entire ring similarly to the domino effect. A series of intramolecular motions were monitored at a temporal resolution of 30 ms and a picometer-level position accuracy by diffracted X-ray tracking (DXT)*1 developed by Yuji Sasaki in 1998. A series of intramolecular motions were predicted using many still images taken by electron microsocpy. Real-time monitoring with DXT has enabled quantitative discussion on the synchronicity of intramolecular motions. In molecular biology, molecules are considered as being stationary with a specific structure. High-speed and high-precision monitoring of a new property of molecules observed in this study, or intramolecular motions, may change the strategies for drug development and the concept of intermolecular interaction in the future. For example, the development of molecules that inhibit only intramolecular motion essential for the expression of a certain function will be more important than the development of drugs based on locks and keys. When the action of new drugs increasingly depends on the target function, the side effects of these drugs can be greatly reduced. Publication: |
<<Glossary>>
*1 Diffracted X-ray tracking (DXT)
DXT is a method of tracking the trajectories of the Laue diffraction spots from nanocrystals with diameters of several 10 nm, which label the target protein molecules. Here, the motion of the nanocrystals coupled with the intramolecular motion of protein molecules is monitored using X-rays by a high-speed and time-sharing method. DXT was designed by Yuji Sasaki in 1997 and he realized his idea in 2000 as a researcher of Precursory Research for Embryonic Science and Technology 21 (PRESTO 21) under the project of Elementary Process and Linkage supported by the Japan Science and Technology Agency (JST). He wrote many papers in journals including Physical Review Letters, Physical Review, BBRC, and Cell. Figure 1 shows the model of structural changes of chaperonin observed in this study.
|
For more information, please contact: |
- Previous Article
- Visualization of Solvated Electrons in Large Cage Structure of Glass (Press Release)
- Current article
- World’s First Discovery of Intramolecular Motions of Chaperonin That Plays an Important Role in Protein Repair (Press Release)