Successful synthesis of an aluminum-based interstitial hydride (Press Release)
- Release Date
- 19 Sep, 2013
- BL14B1 (JAEA Materials Science)
Japan Atomic Energy Agency
Tohoku University
Key research findings
• A new hydride Al2CuH was synthesized by hydrogenation under high-pressure and high-temperature conditions.
• The crystal and electronic structures of Al2CuH illustrated a formation of an interstitial hydride which is a new class of aluminum-based hydride.
|
A joint research group comprising Japan Atomic Energy Agency and Tohoku University succeeded in synthesis of a new aluminum-based interstitial hydride Al2CuH by hydrogenation under high-pressure and high-temperature conditions. This finding will help in expanding the variety of aluminum-based alloy hydrides, which, in turn, will help in developing practical hydrogen-storage materials. Lightweight hydrides with high hydrogen content are desirable for mobile hydrogen-storage applications. Aluminum-based hydrides are promising because of their advantages: low weight, nontoxicity, and the absence of volatile gas products except for hydrogen. Complex aluminum hydrides, whose crystal structures are built of [AlH4]- tetrahedral or [AlH6]3- octahedral units, have been studied widely. However, no complex aluminum hydrides suited for practical applications have been developed yet. If aluminum-based hydrides without such structural units, i.e., interstitial aluminum-based hydrides are synthesized, the results would lead us to expand the variety of aluminum-based hydrides. Such an interstitial aluminum-based hydride was not realized so far. The research group demonstrated that Al2CuH was synthesized by the hydrogenation reaction of Al2Cu alloy under high-pressure and high-temperature conditions. The reaction conditions were explored with the help of in-situ synchrotron radiation X-ray diffraction measurement at BL14B1, SPring-8. The crystal and electronic structures of the hydride were investigated on the basis of powder X-ray diffraction measurement and first-principles calculations, respectively. Both the crystal and the electronic structures illustrated the formation of the interstitial hydride of an aluminum-based alloy. This is the first report of the formation of interstitial aluminum-based hydrides. The present result helps developing other interstitial aluminum-based hydrides for hydrogen storage. Part of this work was supported by New Energy and Industrial Technology Development Organization (NEDO) under “Advanced Fundamental Research Project on Hydrogen Storage Materials”. Publication: |
<<Figures>>
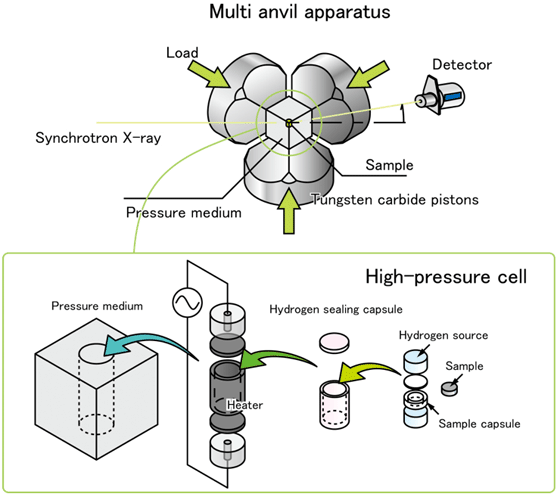
Schematic of the high-pressure apparatus used for the present study. The hydrogenation reaction conditions were explored using the high- pressure apparatus installed on the beamline BL14B1 at SPring-8.
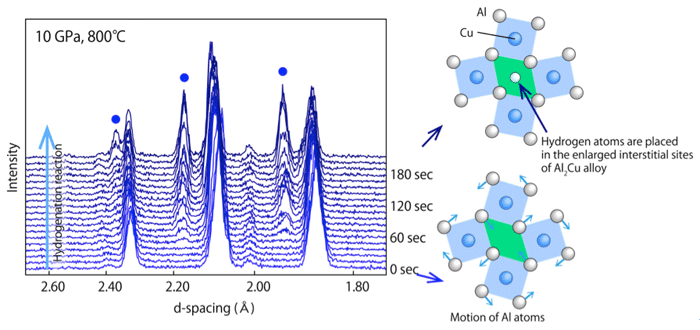
Series of X-ray diffraction profiles taken during the hydrogenation reaction.
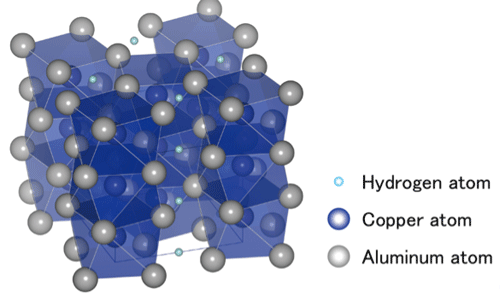
Schematic of the crystal structure of the Al2CuH. Interstitial spaces were enlarged by the hydrogenation reaction to accommodate hydrogen atoms which align linearly in Al2CuH.
|
For more information, please contact: Dr. Yoshinori Katayama (JAEA) Prof. Shin-ichi Orimo (Institute for Materials Research, Tohoku University) |
- Previous Article
- First observation of electronic structure of carbon-related catalysts during fuel cell operation by soft X-ray emission spectroscopy (Press Release)
- Current article
- Successful synthesis of an aluminum-based interstitial hydride (Press Release)