Structure determination of the redox complex which is a key complex in heme metabolism (Press Release)
- Release Date
- 04 Feb, 2014
- BL44XU (Macromolecular Assemblies)
Kurume University
|
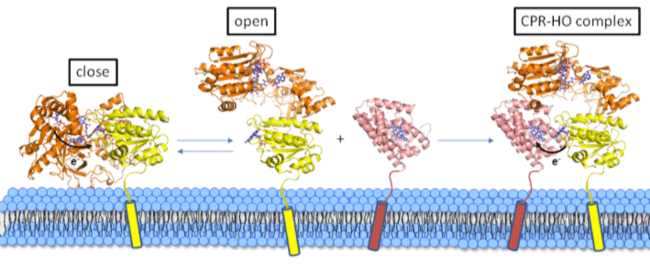
The research group of Assoc. Prof. Masakazu Sugishima and Prof. Masato Noguchi of Kurume University School of Medicine determined the crystal structure of the redox complex of rat NADPH-cytochrome P450 oxidoreductase (CPR) and its redox partner (heme oxygenase; HO). X-ray crystallography(*1) and small-angle X-ray scattering(*2) techniques using synchrotron radiations from SPring-8 and SAGA-LS were applied for the structure determination. As a result, mechanisms how CPR recognizes its redox partners and transfers electrons for enzymatic reactions by redox partners were supposed. The complex structure also gives a basis for the developments of drugs for Antley-Bixler syndrome which is one of the adrenal enzymatic defects, drugs for diseases related to cholesterol and steroid hormone metabolisms, and anticancer drugs. The results was published in Proceeding of the National Academy of Sciences of the United States of America. This work was partly supported by JSPS KAKENHI and by grants from Ishibashi Foundation for the Promotion of Science. Publication: |
<<Figures>>
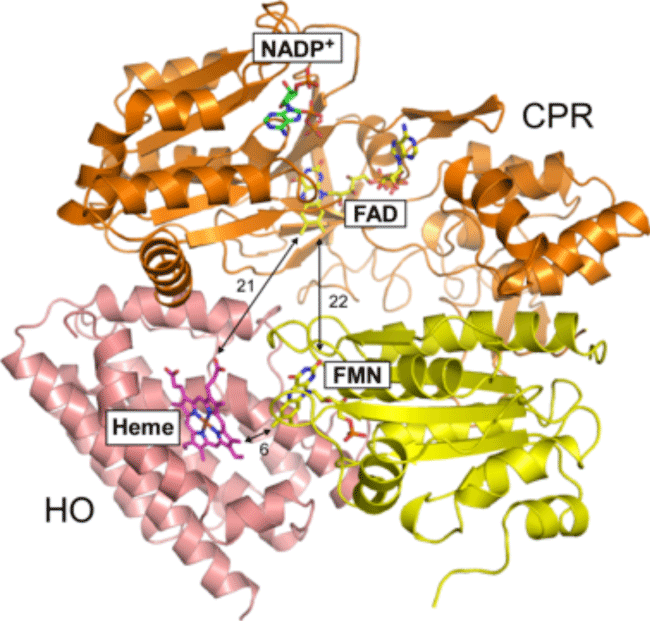
Orange and yellow showed FAD and FMN(*3) domains of CPR, respectively. Pink showed HO. Numerals showed the distances between cofactors in Å(*4) units.
<<Glossary>>
*1 X-ray crystallography
Methods to determine tertiary structures of proteins or the other molecules. Precise tertiary structure can be determined by X-ray irradiation to a monocrystal. The data for the present analysis was collected at the beamline BL44XU, SPring-8.
*2 Small-angel X-ray scattering
Methods to obtain rough structural information of protein. In contrast to X-ray crystallography, this technique is possible to be applied to solution samples. The data for the present analysis was collected at the beamline BL11, SAGA-LS.
*3 FMN, FAD
Coenzymes contained flavin ring. These are biosynthesized from vitamin B2 (riboflavin) and function as cofactors for redox enzymes and blue light receptors.
*4 Å
Units of length. 1 Å is same as 10-10 m (0.1 nm).
|
For more information, please contact: |
- Previous Article
- Successful Synthesis of Artificial Rhodium (Press Release)
- Current article
- Structure determination of the redox complex which is a key complex in heme metabolism (Press Release)