Discovering New Autoimmune Disease Mechanism -Success in x-ray single molecule observations of Autoimmune System- (Press Release)
- Release Date
- 21 Jan, 2015
- BL40XU (High Flux)
University of Tokyo
The Tokyo University of Science (TUS)
Japan Synchrotron Radiation Research Institute (JASRI)
Key points
•Dynamic internal motions of major histocompatibility complex, MHC, a key functional protein responsible for acquired immune responses, with peptides were observed at single molecule level with 1 milliseconds time resolution for the first time in the world.
•It was distinguished the characteristic intramolecular motion of antigenic peptides from MHC by three distinct methods.
•The possible autoimmune peptide had larger internal dynamic motion, and thus produced novel structures that activate certain autoimmune T cells. We propose that regulating internal motion of peptide may become a new methods treating immunological disease.
|
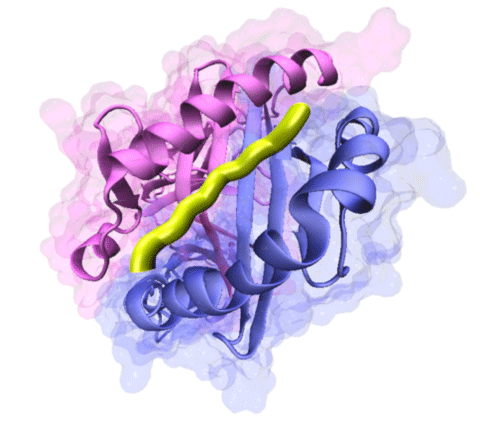
A research group led by Yuji C. Sasaki at the Graduate School of Frontier Sciences, the University of Tokyo, and Haruo Kozono at the Research Institute of Biomedical Sciences, Tokyo Science University, successfully observed three-dimensional (3D) dynamic single-molecule internal motions of peptide/MHC complex, a high-profile protein that controls acquired immune response at a 1 millisecond time resolution and picometer (a length one-hundredth of the diameter of an atom) accuracy for the first time in the world. The group members include Hiroshi Sekiguchi at the Japan Synchrotron Radiation Research Institute, Osami Kanagawa at the International Center for Infectiology Research, INSERM, and Makoto Taiji at the Computational Biology Research Core, Quantitative Biology Center, RIKEN. Publication: |
《Glossary》
*1Major Histocompatibility Complex (MHC)
Major Histocompatibility Complex (MHC) of class II have an open binding groove, which allow various length of bound peptides usually 14-20 amino acids long, although the core part of peptide that buried in the binding groove are nine amino acids. Peptide flanking residue (PFR) are residues outside of binding groove. We found a role of this PFR in thermodynamic sense.
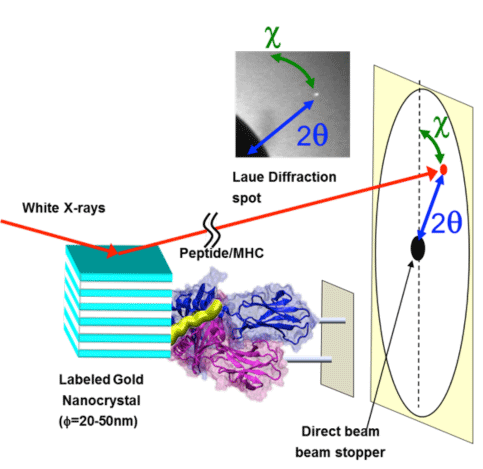
*2Diffracted X-ray tracking (DXT)
DXT is a powerful technique in biological science for detecting atomic-scale dynamic motions of the target protein at the single molecular level at several tens of microsecond time resolution. The dynamics of a single protein can be monitored through trajectory of a Laue spot from a nanocrystal which was attached to the target protein. Prof. Yuji C. Sasaki designed this technique in 1997 and presented it in 2000. He has monitored the internal motions of various proteins and reported the results in many journals (e.g., Physical Review Letters, Physical Review, BBRC, Cell PLoS One, Scientific Reports). The figure shows schematics of the DXT technique applied to a peptide/MHC. A movie is also provided as supplementary material to show the motions of the complex.
*3Intramolecular dynamics in single molecule
In general, intramolecular dynamics is a generic term for the motions of protein molecules that occur upon the expression of their biological functions. In particular, ion channel molecules examined in this study bind to ligands and the central region of the molecules far from the binding site is considered to change greatly. However, this motion has been predicted simply by comparing the stable structural information before and after the structural transitions. An issue for conventional monitoring techniques has been the realization of real-time observations on a time axis because the intramolecular dynamics (motions) of protein molecules is closely related to their functions. In addition, accurately monitoring the motions of individual single molecules requires a single-molecule monitoring technique. The DXT technique was successfully applied to internal single-molecule monitoring for the first time in the world.
|
For more information, please contact: Associate Professor Haruo Kozono (The Tokyo University of Science) |
- Previous Article
- Dark magma at the bottom of the lower mantle -New insights into the super-hot plumes in the deep Earth- (Press Release)
- Current article
- Discovering New Autoimmune Disease Mechanism -Success in x-ray single molecule observations of Autoimmune System- (Press Release)