Establishment of new method of evaluating performance of lithium-ion batteries using high-energy X-ray scattering (Press Release)
- Release Date
- 31 Aug, 2017
- BL08W (High Energy Inelastic Scattering)
August 31, 2017
Japan Synchrotron Radiation Research Institute
Gunma University
Ritsumeikan University
Kyoto University
Key research findings
・Electron orbitals that determine the performance of lithium-ion batteries were visualized by high-energy X-ray scattering experiments and theoretical calculation.
・The possibility of monitoring the potential shift in batteries was proven by analyzing scattered X-rays.
・This research achievement is expected to contribute to lithium battery diagnosis and improved performance.
A research group with members from Japan Synchrotron Radiation Research Institute, Gunma University, Ritsumeikan University, and Kyoto University has successfully visualized the electron orbitals that determine the performance of batteries by a combination of experiments using high-brilliance high-energy synchrotron radiation X-rays at a large synchrotron radiation facility, SPring-8*1, and theoretical calculation. This achievement was made jointly with a theoretical research group with members from Northeastern University (US), University of Antwerp (Belgium), and AGH University of Science and Technology (Poland). In lithium-ion batteries, a current flows when the conduction electrons of lithium move to the cathode material. The electron orbitals into which the electrons are received by the cathode material are called the redox orbitals.*2 These orbitals are known to be an important index of battery characteristics, such as potential and capacity. However, the state of the redox orbitals has remained unclear because of the difficulty of experimentation. The joint research group performed Compton scattering*3 measurements using high-energy X-rays (≥100 keV) on olivine lithium iron phosphate, which is used as a safe, high-performance cathode material in batteries. In combination with a high-reliability theoretical calculation, the measurements led to the successful visualization of redox orbitals from polycrystalline olivine lithium iron phosphate. As a result, it was found that the redox orbitals greatly depend on the degree of crystal distortion of the cathode material and that the change in the state of the redox orbitals is proportional to the change in potential, an important index of battery characteristics. By applying these findings, the potential shift can be monitored by Compton scattering measurements using high-energy X-rays. Compton scattering measurements enable the nondestructive observation of the inside of lithium-ion batteries because of the high penetrability of the high-energy X-rays used. Conventionally, Compton scattering has been used to measure the distribution of lithium-ion concentration. The results of this study indicate that the electron state distribution in the redox orbitals and the distribution of the local potential shift can also be measured by Compton scattering. The operating principle of lithium-ion batteries can thus be understood from a more fundamental viewpoint, contributing to the improved performance of lithium-ion rechargeable batteries. The achievements of this research were published in Science Advances, an open-access journal of the American Association for the Advancement of Science (AAAS), which also publishes Science. This research was partly supported by the program for the development of advanced measurement and analysis systems of the Japan Science and Technology Agency (JST) as well as Grants-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science, entitled “Non-destructive quantitation method for lithium using Compton scattering (Principal Investigator: Assistant Professor Kosuke Suzuki)” and “Clarification of reaction mechanism behind battery electrodes for the development of in-operando quantitation of Li concentration (Principal Investigator: Assistant Professor Kosuke Suzuki)”. Reference |
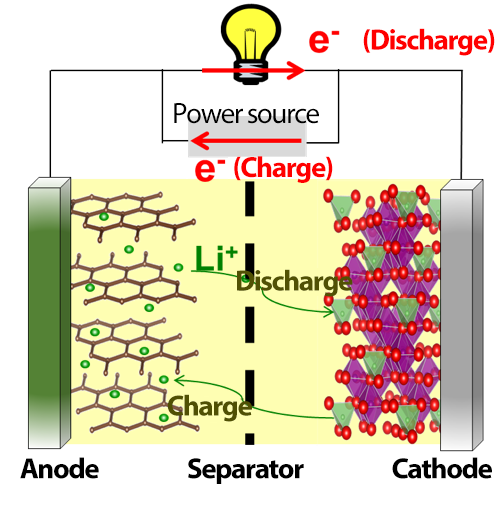
Fig. 1 Lithium-ion batteries charge and discharge when lithium ions are transferred between the cathode and the anode. Lithium–transition-metal composite oxides are used for the cathode, whereas carbon materials are used for the anode.
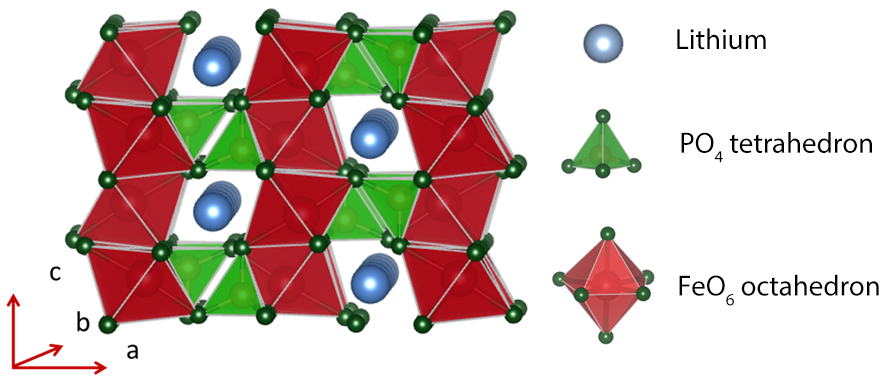
Fig. 2 Crystalline structure of olivine lithium iron phosphate cathode material.
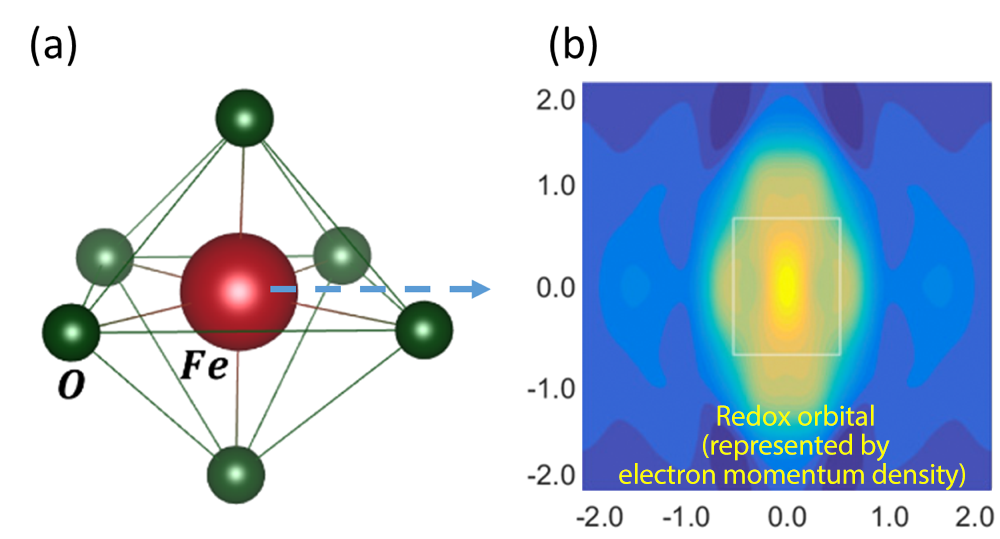
Fig. 3 (a) Steric structure of FeO6 octahedron of olivine lithium ion phosphate and (b) momentum map of redox orbital of iron (Fe) at the center of the FeO6 octahedron. Both the ordinate and abscissa represent the momentum of electrons, that is, the magnitude of velocity. The rectangle in the figure represents the first Brillouin zone.
<Glossary>
※1 SPring-8
SPring-8 is the world’s largest synchrotron radiation facility, located in Harima Science Park, Hyogo Prefecture, Japan. It is managed by RIKEN and operated by JASRI. Synchrotron radiation refers to narrow and powerful electromagnetic waves that are produced when electrons are accelerated to nearly the speed of light and their traveling direction is bent by electromagnets. Synchrotron radiation from SPring-8 is widely used for nanotechnological, biotechnological, and industrial studies.
※2 Redox orbital
Chemical reactions in which electrons are transferred between atoms, ions, or substances are called redox reactions. When electrons are transferred between atoms, ions, or substances during redox reactions, the electrons are received by electron orbitals, which are called the redox orbitals.
※3 Compton scattering
Light (X-rays) can have the nature of particles called photons. When X-ray photons and electrons collide with each other, the photons are scattered by the electrons, similarly to billiard balls, and the electrons are expelled. Therefore, the energy of the photons after collision is observed to be lower than that before collision. Such scattering is called Compton scattering. In many textbooks, Compton scattering is explained as elastic collisions between stationary electrons and X-ray photons. However, electrons in actual materials are in constant motion. Therefore, the scattered X-ray photons have an energy distribution that reflects the momentum of electrons (the Doppler effect). The scattering intensity of X-rays with respect to energy is called the Compton profile, which reflects the momentum of the electrons in materials and is used to examine the electron state of the materials.
Contact |
- Previous Article
- High-speed switching for ultrafast electromechanical switches and sensors (Press Release)
- Current article
- Establishment of new method of evaluating performance of lithium-ion batteries using high-energy X-ray scattering (Press Release)
 spring8.or.jp
spring8.or.jp