New Strategy for Prevention of Iron Deficiency Anemia: Understanding the role of vitamin C in enhancing iron absorption in human intestine (Press Release)
- Release Date
- 20 Aug, 2018
- BL32XU (RIKEN Targeted Proteins)
August 20, 2018
University of Hyogo
RIKEN
Shimane University
University of Maryland
University of British Colombia
Iron is required in a variety of important biological processes including oxygen transport and storage, redox reactions, DNA biosynthesis, energy production, and so on; therefore, all organisms must maintain sufficient amounts of iron in cells. Body iron (approximately 5 g in adult) is maintained from daily dietary iron intake. It is known the dietary iron is absorbed on duodenal villi in humans, but the detail molecular mechanisms are unclear. The collaborative research team led by Dr. Hitomi Sawai (Graduate School of Life Science, University of Hyogo) and Dr. Hiroshi Sugimoto (RIKEN SPring-8 Center) solved the molecular structures of a key membrane-integrated protein for dietary iron absorption in human duodenum. The researches revealed for the first time how vitamin C enhances iron absorption at the atomic level. The results of this study will be released on August 17, 2018 (05:00 EST) in the international scientific journal “Communications Biology” published by Nature Publishing Group. Information of the journal publication |
Background of research
Iron metabolism is finely regulated in humans. A healthy adult contains approximately 5 g iron, where it is needed for making red blood cells or stored in liver or distributed in various proteins involved in human biological processes. Apart from this, 1 – 2 mg of iron is loss every day, through skin and minor blood losses. This loss is balanced by 1 – 2 mg of daily dietary iron intake. The failure to compensate this loss lead to iron deficiency anemia, the most widespread micronutritional disorder worldwide today. Heme [1] is most easily absorbed, but it only accounts for 40% of iron derived from meat. The balance 60% comes in the form of non-heme iron. Also, all vegetables, grains, and most sea food and iron fortified food contain solely iron ion. Hence iron absorption plays a crucial role in maintaining human iron homeostasis, and reduced the absorption cause severe iron deficiency problems especially in vegans and children.
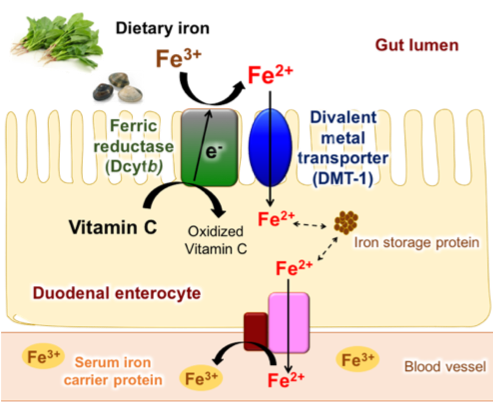
The iron ion naturally occurs in ferric (Fe3+) form and is transported from gut lumen into duodenal enterocyte by Divalent Metal Transporter-1 (DMT-1), which only favors the transport of ferrous (Fe2+) iron. Therefore, the reduction of Fe3+ to Fe2+ is the key step in iron absorption. For the reduction from Fe3+ to Fe2+, duodenal cytochrome b (Dcytb) works on the cell membrane of duodenal enterocyte. The Dcytb transfers an electron from vitamin C [2] on the cytoplasmic side to the Fe3+ on the gut lumen side, thereby reducing Fe3+ to Fe2+ (Fig. 1). Our research group aimed to clarify the detailed molecular mechanisms of Fe3+ reduction by Dcytb.
Fe3+ from dietary source is reduced to Fe2+ by Dcytb. Reduced Fe2+ is transported into duodenal enterocyte by DMT-1. The transported iron ions are either stored in iron storage protein or transported into blood vessel to be carried to other parts of body.
Research methods and outcomes
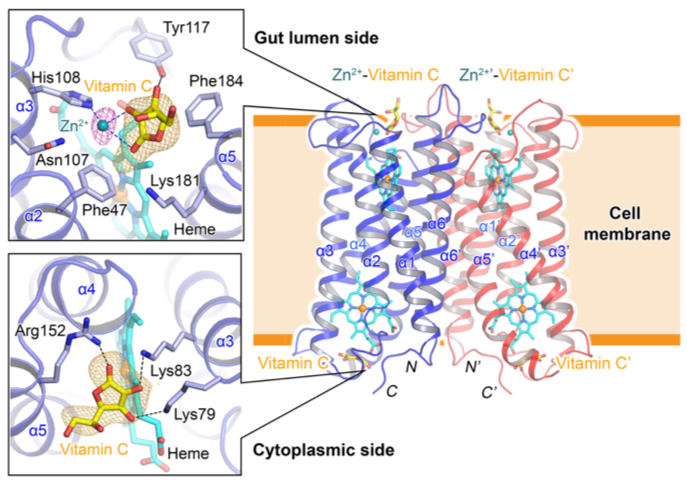
To understand the mechanism of Fe3+ reduction by Dcytb, the structure in the presence of vitamin C and metal ion was solved by X-ray crystallography [3] using crystals grown with lipidic cubic phase technique [4]. X-ray crystallographic data collection was carried out in RIKEN microfocus beamline BL32XU at SPring-8 [5]. The overall structure of Dcytb appeared as a homodimer [6], containing two heme molecules in each monomer (Fig. 2). The binding sites of vitamin C and metal ion (Zn2+, alternative to Fe3+) were obtained from the solved structure. This provided crucial information about the mechanism and the importance of vitamin C in Fe3+ reduction.
In this structure, three positively charged amino acid residues (two lysine and one arginine) form hydrogen bonds with vitamin C on the cytoplasmic side (Fig. 2). This structural conformation is reasonable with the function of vitamin C as an electron donor for Dcytb. The most significant finding from the structure is the metal ion binding site on the gut lumen side of Dcytb. Based on the structure, the metal ion cooperatively binds with vitamin C. This arrangement means that metal ion binding to Dcytb is stabilized by the presence of vitamin C. The binding of vitamin C alongside Fe3+ on the gut lumen side of Dcytb can be understood by knowing the relationship between the Fe3+ solubility and physiological pH. Fe3+ is soluble at the low pH present in stomach, but as the pH increases upon passage into the duodenum, precipitates form unless iron is bound by soluble iron chelators. Thus, vitamin C on the gut lumen side acts as an iron chelator to maintain Fe3+ solubility and support Fe3+ binding to Dcytb. The vitamin C also mediates the electron transfer from heme in the gut lumen side to Fe3+, thereby reducing Fe3+ to Fe2+, enabling Fe2+ to be transported into duodenal enterocyte by DMT-1. Another significant finding in the present study is that, not only vitamin C but also citric, malic, and oxalic acids can assist metal-binding to the gut lumen side of Dcytb as Fe3+-chelotors. Our functional assay with those dietary organic acids promote Fe3+ reduction by Dcytb at duodenal pH.
Dcytb is a homodimer, with two heme molecules (cyan sticks) in each monomer. Each monomer consists of 6 transmembrane helices (α1 - α6), with both N and C terminal on the cytoplasmic side. Vitamin C is bound to cytoplasmic side of Dcytb by forming hydrogen bonds with two lysine (Lys79 and Lys83) residues and one arginine (Arg152) residue. The binding of Zn2+ - vitamin C complex was observed on the gut lumen side. Zn2+ cooperatively coordinates with vitamin C.
Social significance and future prospects of this study
Iron deficiency continues to be a significant cause of malnutrition in both developing and industrialized nations, partly because intestinal absorption of dietary iron is relatively inefficient. The structural information of Dcytb can facilitate the development of new, structure-based strategies for promoting the reduction of dietary iron and thereby enhancing the bioavailability of this essential nutrient. By using the elucidated information on Dcytb, it is possible to develop a new guideline for prevention of iron deficiency anemia, which affects approximately 30% of world population (more than 2 billion).
Glossary
[1] Heme:
A compound containing an iron atom (heme iron) in the center of the planar cyclic ring called porphyrin. Porphyrins are classified into several groups depending on the type and place of ring modification. Hemoproteins are defined as proteins that are functional only after incorporating a heme molecule, and in general, they appear red. Representatives of hemoproteins are hemoglobin, which delivers oxygen, cytochromes in the electron transport system, and peroxidases, which functions as an enzyme. Dcytb in this study is also hemoprotein, and heme is used to mediate the electron transfer from cytoplasmic to apical side.
[2] Vitamin C:
A water-soluble vitamin, also known as L-ascorbate. It is not biosynthesized in human body and mainly obtained from diet. In Dcytb, vitamin C works as a physiological reductant.
[3] X-ray crystallography:
A technique used to determine the structures of molecules. This is achieved by using a beam of short-wavelength X-rays to strike a crystal with and orderly arrangement of atoms and carefully analyzing the intensity of the scattered X-rays. The three-dimensional structured of a variety of biological molecules have been determined by this method.
[4] Lipidic cubic phase technique:
One of the crystalline phases that form upon mixing lipids with water (protein) at suitable conditions. This technique provides a more native like cell membrane environment for crystallization of membrane proteins.
[5] SPring-8:
It is the world’s largest synchrotron radiation facility, located in Harima Park City in Hyogo Prefecture. This facility is managed by RIKEN and is capable of delivering what is now the strongest radiation in the world. At SPring-8 which stands for Super Photon rin-8 GeV (= 8 billion electron volts), waves of electromagnetic radiation are generated by accelerating electrons to near the speed of light and then bending the direction of electrons using a magnetic field. Electromagnetic radiation generated in this way is called synchrotron radiation, which has been widely used for research in a wide range of disciplines and industries, including nanotechnology and biotechnology.
[6] Homodimer:
An oligomeric state in which two molecules are assembled by physical and chemical action. The dimer of the same molecule is called homo-dimer, the dimer by heterologous molecules is called the hetero-dimer. The oligomeric state of Dcytb in this study is a homo-dimer.
Research group
Cellular Regulation Laboratory, Graduate School of Life Sciences, University of Hyogo, Japan
Prof. Yoshitsugu Shiro, Dr. Hitomi Sawai, Ms. Menega Ganasen, Ms. Hanae Takeda, Ms. Honami Asakura
RIKEN SPring-8 Center, Japan
Dr. Hiroshi Sugimoto, Dr. Takehiko Tosha, Dr. Kunio Hirata, Dr. Keitaro Yamashita, Dr. Hiromi Togashi
Department of Biochemistry, Shimane University School of Medicine, Japan
Dr. Takashi Urano, Ms. Yuko Nariai
Department of Animal and Avian Sciences, University of Maryland, United States of America
Prof. Iqbal Hamza, Dr. Xiaojing Yuan
Department of Biochemistry and Molecular Biology and Centre for Blood Research, University of British Columbia, Canada
Prof. A. Grant Mauk
Financial supports
This work was supported by the Japan Society for the Promotion of Science KAKENHI Grant numbers JP26220807, JP24687015, JP18H02396, JP25871213 and JP18K05321, the Fumi Yamamura Memorial Foundation for Female Natural Scientists from Chuo Mitsui Trust and Banking, Hyogo Science and Technology Association, RIKEN Pioneering Projects “Integrated Lipidology”, “Molecular System” and “Fundamental Principles Underlying the Hierarchy of Matter”.
Contacts Dr. Hiroshi Sugimoto |
- Current article
- New Strategy for Prevention of Iron Deficiency Anemia: Understanding the role of vitamin C in enhancing iron absorption in human intestine (Press Release)
 sci.u-hyogo.ac.jp
sci.u-hyogo.ac.jp