Reason for tooth-decay prevention efficacy of chewing gum
Prevention of tooth decay
Tooth decay is one of the most common diseases. Although tooth decay is not a life-threatening disease, treatment procedures for tooth decay are an unpleasant experience. Thus far, oral hygiene measures, such as regular tooth brushing, have been widely carried out to prevent tooth decay. In recent years, the development of foods for specified health use (FOSHUs)*1, which contribute to the maintenance and improvement of dental health, has been promoted. Chewing gum is attracting attention as a FOSHU.
Tooth decay is caused by acids produced by oral bacteria from carbohydrates, leading to the erosion of tooth enamel. Dental plaque is a visible sign of tooth decay, resulting from the buildup of food debris, saliva, and bacteria on the tooth surface. Regular tooth brushing removes dental plaque.
The effect of brushing is further improved using toothpaste. In particular, toothpastes containing fluorine or hydroxyapatite*2 have a protective coating effect on teeth. When dental plaque is left unremoved, it builds up on the surface of teeth and becomes a dental calculus, which is difficult to remove by simple brushing.
Regeneration of enamel by chewing gum
In 2003, a chewing gum with a tooth-decay preventive effect became commercially available. The chewing gum was approved as a FOSHU and contained a raw material of hydroxyapatite called phosphoryl-oligosaccharide calcium (POs-Ca®, Ezaki Glico Co., Ltd.*3), which is produced from potato starch, and conventionally considered as a byproduct of glucose production.
Ezaki Glico Co., Ltd. has focused on phosphoryl-oligosaccharide calcium since 1995. A research group led by Hiroshi Kamasaka, the manager of the Health Science Laboratory, Ezaki Glico Co., Ltd., carried out various evaluation tests using potatoes produced in Hokkaido, which have the highest amounts of the raw material phosphoryl-oligosaccharide calcium, and found that phosphoryl-oligosaccharide calcium contributes to dental health maintenance.
Before explaining the effect of phosphoryl-oligosaccharide calcium, we must understand the mechanism underlying tooth decay. Here, the key words are decalcification and remineralization.
As shown in Fig. 1, a tooth is composed of the enamel, dentine, and pulp from the surface. The strength of the enamel is maintained by aligned fine hexagonal cylindrical crystals of hydroxyapatite with dimensions of about 20 nm (a nanometer is one-billionth of a meter) diameter and about 100 nm length. Tooth decay indicates a condition in which a tooth cavity develops in the enamel, and is caused by the decalcification of calcium phosphate in the enamel.
At the initial stage of tooth decay, mineral components inside a tooth, not on the upper surface, are lost. Although no cavity (substance defect) is found on the surface of the enamel by visual inspection, a white opaque area appears, which is called initial caries and strictly speaking is in a stage immediately prior to tooth decay. At this stage, it may be possible for a tooth to restore its healthy condition by remineralization, by which phosphate and calcium ions are supplemented through the effect of saliva and other factors. Phosphoryl-oligosaccharide calcium supports remineralization.
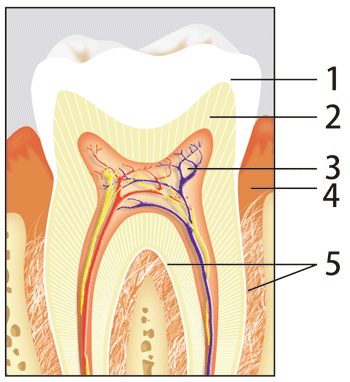
Fig. 1 Structure of tooth.
1.Enamel, 2.Dentine, 3.Pulp, 4.Gum, 5.Cementum
How many crystals are regenerated?
Remineralization in the presence of phosphoryl-oligosaccharide calcium has been examined by transversal microradiography (TMR) to measure the changes in mineral components, morphological observation using an electron microscope, and elemental analysis. However, these methods reveal only the mineral contents, and the structural changes of crystals, which are a key to maintaining tooth strength, have not been examined in detail.
Dr. Tomoko Tanaka of the Health Science Laboratory, Ezaki Glico Co., Ltd. addressed the challenge of measuring the crystallinity of a tooth that is remineralized in the presence of phosphoryl-oligosaccharide calcium. Dr. Tanaka said that the Synchrotron Radiation Industrial Applications Seminar held at the end of 2006 encouraged her to use the SPring-8 beamline for measurement. When she consulted with a coordinator of the seminar, she was introduced to Dr. Naoto Yagi, a chief scientist of Japan Synchrotron Radiation Research Institute (JASRI) to discuss the details of the study method.
Bovine teeth were used as samples, because human teeth have large differences among individuals. Bovine tooth samples were cut into blocks and embedded in resin and other materials. Each sample was cut into three pieces, corresponding to unaffected, decalcified, and remineralized areas (Fig. 2).
The diameters of a regular beam (20-30 μm) are too large because the thickness of a decalcified area is as small as 150 μm (a micrometer is one-millionth of a meter). Therefore, the synchrotron radiation X-rays of BL40XU with a beam diameter of approximately 6 μm were used. Crystal structure changes and alignment were examined by wide-angle X-ray scattering, and the gap between crystals was measured by small-angle X-ray scattering (Fig. 3). Dr. Tanaka said that Dr. Yagi considerably supported her research and that her achievements would not have been possible without his help.
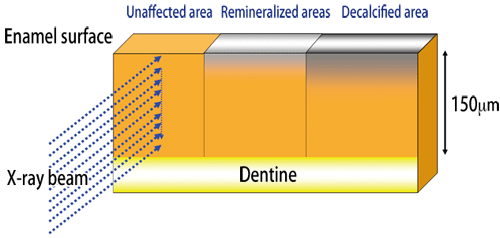
Fig. 2 Preparation of sample used for measurement at SPring-8.
A gel-like material is placed on the sample, and drops of acid solution is placed on the sample surface. Decalcification occurs over two weeks. Remineralization by POs-Ca® occurs over at least 12 hours.
Fig. 3 Dr. Tanaka setting a bovine sample onto the apparatus.
X-ray beams are irradiated from the right side and the detector on the left is used for measurement.
What can a chewing gum do to prevent tooth decay?
From the measurements, two findings were obtained. Decalcification was found to occur at not only the atomic level but also the crystal structure level. During remineralization, enamel crystals are regenerated at the crystal structure level; the crystals in a remineralized area align similarly to those in an unaffected area (Fig. 4). On the basis of these findings obtained at SPring-8, Dr. Tanaka speculated the process of remineralization by phosphoryl-oligosaccharide calcium as shown in Fig. 5.
A chewing gum using POs-Ca® has been continuously improved since its first release in the market. In August 2008, the product was completely modified (Fig. 6). The mixing ratio of flavors and other factors were changed to significantly improve the taste and make it last for a long time, and the packaging of the product and the shape and size of the gum were modified to attract consumers'interest as a common food product.
Dr. Tanaka pointed out the following two future research themes: collection of data on human teeth for clinical applications and collection of data based on which the value of chewing gum can be easily explained to consumers. Ezaki Glico Co., Ltd. is exploring new possibilities of phosphoryl-oligosaccharide calcium (POs-Ca®) and dental gum.
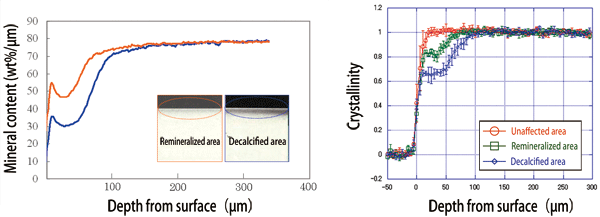
Fig. 4 Remineralization by POs-Ca® (left) and effect of recrystallization (right).
Left: Mineral content decreases on the surface owing to decalcification (blue); however, it is restored by remineralization (orange).
Right: Hydroxyapatite crystals originally existing in the unaffected area (red) are lost in the decalcified area (blue). The hydroxyapatite crystals, as well as crystallinity, are regenerated by POs-Ca® in the remineralized area (green).
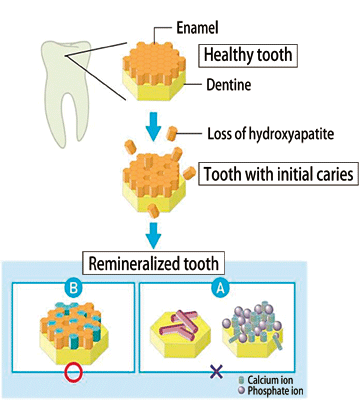
Fig. 5 Expected model of remineralization by POs-Ca®.
The components of enamel are not discretely regenerated (A), but rather restored simultaneously with the recovery of crystallinity.

Fig. 6 New product “POs-Ca (Flat Style)”®.
Column: If I won the lottery, I would buy three cars.

Dr. Tanaka is a car enthusiast, and said if she won the lottery, she would buy three cars. The interviewer does not know how much she would like to spend on one car, but she was really impressed by the Lego Ferrari® presented at her wedding reception. When she has free time during the weekend, she drives to a tennis court to play tennis, which is her other hobby.
Although she said that she was embarrassed when the seller of bovine teeth asked her, “For one who is working for Ezaki Glico, why do you need bovine teeth?”, she purchased the teeth anyway. Similar to her hobbies, her attitude of making every effort to achieve her goal is outstanding. She said, “Although we found that enamel recrystallization actually occurs, the detailed mechanism underlying recrystallization remains to be clafified. I would really like to discover the mechanism.” She is really enthusiastic about science.
Interview and writing by Tomoaki Yoshito (Sci-Tech communications Incorporated)
Glossary
*1 Food for specified health use (FOSHU)
Foods undergoing further examination on the basis of experimental data and whose efficacies are approved as valuable to be listed on the package by the Ministry of Health, Labour and Welfare. These foods are also known as Tokuho.
*2 Hydroxyapatite
Hydroxyapatite is a principal component of teeth and bones. It is used as a material of artificial bones in surgical treatment. It has the chemical structural formula Ca5(PO4)3(OH).
*3 Phosphoryl-oligosaccharide calcium (POs-Ca®)
Phosphoryl-oligosaccharide calcium has a high solubility of 70% or more at room temperature, and is highly soluble in saliva. According to Dr. Tanaka, it is bitter and salty.
This article was written following an interview with Dr. Tomoko Tanaka of the Health Science Laboratory, Ezaki Glico Co., Ltd.