Local structure analysis of oxygen storage promoters, CeO2-ZrO2
問い合わせ番号
SOL-0000001206
ビームライン
BL01B1(XAFS I)
学術利用キーワード
| A. 試料 | 無機材料 |
|---|---|
| B. 試料詳細 | 結晶性固体, 非晶質、ガラス |
| C. 手法 | 吸収、及びその二次過程 |
| D. 手法の詳細 | XAFS, EXAFS |
| E. 付加的測定条件 | 偏光(直線), 室温 |
| F. エネルギー領域 | X線(4~40 keV), X線(>40 keV) |
| G. 目的・欲しい情報 | 化学状態, 結合状態, 局所構造, 機能構造相関, 機能発現 |
産業利用キーワード
| 階層1 | 機械, 金属, 環境, 化学製品, 工業材料, その他 |
|---|---|
| 階層2 | 触媒 |
| 階層3 | |
| 階層4 | 局所構造, 化学状態 |
| 階層5 | XAFS, NEXAFS |
分類
A80.34 触媒化学, M40.10 XAFS
利用事例本文
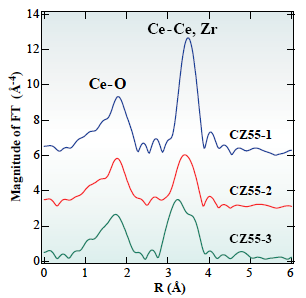
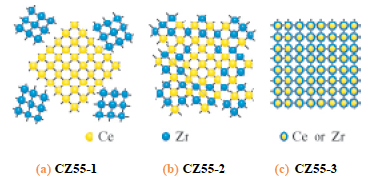
In this solution, EXAFS method at Ce and Zr K-edges was applied to study local structure of CeO2-ZrO2 mixed oxide, oxygen storage promoters in automotive catalysts. The EXAFS method is a powerful technique to study local structure (distance, coordination number, species of neighbor atoms) of selected elements both in crystalline states and in non-crystalline states. Figure 1 shows radial structure function of Te atom after Fourier transform of Ce K-edge EXAFS spectra. Simultaneous analysis of EXAFS data set at 2 absorption edges revealed that homogeneous CeO2-ZrO2 solid solution (Fig. 2(c)) has high oxygen storage capacity.
Fig. 1 Radial structure function for Ce atom in CeO2-ZrO2 samples prepared by different methodologies.
Fig.2 Model structures of samples in Fig. 1.
[ Y. Nagai, T. Yamamoto, T. Tanaka, S. Yoshida, T. Nonaka, T. Okamoto, A. Suda and M. Sugiura, Journal of Synchrotron Radiation 8, 616-618 (2001), Fig. 3, 4, 6,
©2001 Internationa Union of Crystallography ]
画像ファイルの出典
所内報
誌名
SPring-8 Research Frontiers, 1999-2000
ページ
47, 48
測定手法
XAFS spectra of dense samples are taken in a transmission mode. This method is performed by measuring x-ray absorption as a function of x-ray energy around an absorption edge of a selected element. For elements from Sn to Eu in the periodic table, K-edge XAFS has advantages, such as high-spatial resolution and small contribution of multi-electron excitations, compared with L3-edge XAFS. Acquisition time per XAFS spectrum using QXAFS mode is 10-15 min in this solution.
画像ファイルの出典
私信等、その他
詳細
講習会プレゼン資料
測定準備に必要なおおよその時間
2 時間
測定装置
| 装置名 | 目的 | 性能 |
|---|---|---|
| XAFS Measurement System | Measurement of XAFS spectra | 3.8-113 keV |
| Ionization Chamber | Measurement of transmission mode XAFS | concentration > 1000 ppm |
参考文献
| 文献名 |
|---|
| Y. Nagai et al., Catalysis Today, 74, (2002) 225 |
関連する手法
アンケート
SPring-8だからできた測定。他の施設では不可能もしくは難しい
本ビームラインの主力装置を使っている
測定の難易度
初心者でもOK
データ解析の難易度
中程度
図に示した全てのデータを取るのにかかったシフト数
1シフト以下