Local structure analysis of hydroxyapatites-bound Pd catalysis
問い合わせ番号
SOL-0000001208
ビームライン
BL01B1(XAFS I)
学術利用キーワード
| A. 試料 | 無機材料 |
|---|---|
| B. 試料詳細 | 非晶質、ガラス |
| C. 手法 | 吸収、及びその二次過程 |
| D. 手法の詳細 | XAFS, EXAFS, XANES |
| E. 付加的測定条件 | 偏光(直線), 室温 |
| F. エネルギー領域 | X線(4~40 keV) |
| G. 目的・欲しい情報 | 化学状態, 結合状態, 局所構造, 機能構造相関, 機能発現 |
産業利用キーワード
| 階層1 | 化学製品, 工業材料, その他 |
|---|---|
| 階層2 | 触媒, 日用品(シャンプー,化粧品,歯磨き粉など) |
| 階層3 | |
| 階層4 | 局所構造, 化学状態 |
| 階層5 | XAFS |
分類
A80.34 触媒化学, M40.10 XAFS
利用事例本文
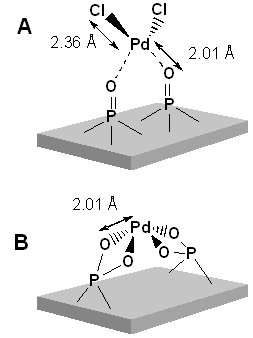
In this solution, fluorescence EXAFS method at Pd K-edge was applied to hydroxyapatites (HAP)-bound Pd (0.16 wt%) catalysis (PdHAP). For dilute samples, the fluorescence EXAFS method is a powerful technique to study local structure (distance, coordination number, species of neighbor atoms) of selected elements both in crystalline states and in non-crystalline states. The PdHAP have demonstrated different catalytic activities, such as aerobic alcohol oxidation and Heck reaction, only by controlling the ratio of Ca:P in the parent HAP. EXAFS analysis revealed that local structure of PdHAP strongly depends on composition of HAP and causes heterogeneous catalytic activities as shown in the figure.
Fig. Model structure of PdHAP catalysts for aerobic alcohol oxidation (a) and Heck reaction (b).
[Journal of the American Chemical Society 124, 11572-11573 (2002), Fig. 1(b), ©2002 The American Chemical Society.]
[ K. Mori, K. Yamaguchi, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, Journal of the American Chemical Society 124, 11572-11573 (2002), Fig. 1(b),
©2002 American Chemical Society ]
画像ファイルの出典
原著論文/解説記事
誌名
K. Mori et al., J. American Chem. Soc., 124, (2002) 11572-11573
図番号
1(b)
測定手法
XAFS spectra of dilute samples are taken by fluorescence XAFS method. This method is performed by measuring fluorescence from excited atoms as a function of x-ray energy around an absorption edge of a selected element. A 19-element Ge detector is used for measurement of 1-1000 ppm samples. Acquisition time per XAFS spectrum is 1-1.5 hr in this solution.
画像ファイルの出典
図なし
測定準備に必要なおおよその時間
4 時間
測定装置
| 装置名 | 目的 | 性能 |
|---|---|---|
| XAFS Measurement System | Measurement of XAFS spectra | 3.8-113 keV |
| 19 Ge Detector | Measurement of XAFS spectra of dilute sample and thin film | concentration: 1-1000 ppm, thickness > 0.1 nm |
参考文献
| 文献名 |
|---|
| K. Mori et al., J. American Chem. Soc., 124, (2002) 11572 |
関連する手法
アンケート
本ビームラインの主力装置を使っている
測定の難易度
熟練が必要
データ解析の難易度
中程度
図に示した全てのデータを取るのにかかったシフト数
1シフト以下