Local structure analysis of oxygen storage promoters, CeO2-ZrO2
Inquiry number
SOL-0000001206
Beamline
BL01B1 (XAFS I)
Scientific keywords
| A. Sample category | inorganic material |
|---|---|
| B. Sample category (detail) | solid-state crystal, amorphous, glass |
| C. Technique | absorption and its secondary process |
| D. Technique (detail) | XAFS, EXAFS |
| E. Particular condition | polarization (linear), room temperature |
| F. Photon energy | X-ray (4-40 keV), X-ray (> 40 keV) |
| G. Target information | chemical state, chemical bonding, local structure, function and structure, function |
Industrial keywords
| level 1---Application area | mechanics, environment, Chemical product, industrial material, others |
|---|---|
| level 2---Target | catalysis |
| level 3---Target (detail) | |
| level 4---Obtainable information | local structure, chemical state |
| level 5---Technique | XAFS, NEXAFS |
Classification
A80.34 catalysis, M40.10 XAFS
Body text
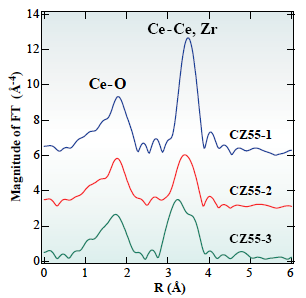
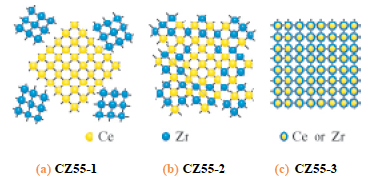
In this solution, EXAFS method at Ce and Zr K-edges was applied to study local structure of CeO2-ZrO2 mixed oxide, oxygen storage promoters in automotive catalysts. The EXAFS method is a powerful technique to study local structure (distance, coordination number, species of neighbor atoms) of selected elements both in crystalline states and in non-crystalline states. Figure 1 shows radial structure function of Te atom after Fourier transform of Ce K-edge EXAFS spectra. Simultaneous analysis of EXAFS data set at 2 absorption edges revealed that homogeneous CeO2-ZrO2 solid solution (Fig. 2(c)) has high oxygen storage capacity.
Fig. 1 Radial structure function for Ce atom in CeO2-ZrO2 samples prepared by different methodologies.
Fig.2 Model structures of samples in Fig. 1.
[ Y. Nagai, T. Yamamoto, T. Tanaka, S. Yoshida, T. Nonaka, T. Okamoto, A. Suda and M. Sugiura, Journal of Synchrotron Radiation 8, 616-618 (2001), Fig. 3, 4, 6,
©2001 Internationa Union of Crystallography ]
Source of the figure
Bulletin from SPring-8
Bulletin title
SPring-8 Research Frontiers, 1999-2000
Page
47, 48
Technique
XAFS spectra of dense samples are taken in a transmission mode. This method is performed by measuring x-ray absorption as a function of x-ray energy around an absorption edge of a selected element. For elements from Sn to Eu in the periodic table, K-edge XAFS has advantages, such as high-spatial resolution and small contribution of multi-electron excitations, compared with L3-edge XAFS. Acquisition time per XAFS spectrum using QXAFS mode is 10-15 min in this solution.
Source of the figure
Private communication/others
Description
講習会プレゼン資料
Required time for experimental setup
2 hour(s)
Instruments
| Instrument | Purpose | Performance |
|---|---|---|
| XAFS Measurement System | Measurement of XAFS spectra | 3.8-113 keV |
| Ionization Chamber | Measurement of transmission mode XAFS | concentration > 1000 ppm |
References
| Document name |
|---|
| Y. Nagai et al., Catalysis Today, 74, (2002) 225 |
Related experimental techniques
Questionnaire
The measurement was possible only in SPring-8. Impossible or very difficult in other facilities.
This solution is an application of a main instrument of the beamline.
Ease of measurement
Easy
Ease of analysis
Middle
How many shifts were needed for taking whole data in the figure?
Less than one shift