State-selective XAFS spectroscopy
Inquiry number
SOL-0000001041
Beamline
BL39XU (X-ray Absorption and Emission Spectroscopy)
Scientific keywords
| A. Sample category | inorganic material |
|---|---|
| B. Sample category (detail) | metal, alloy, semiconductor, superconductor, magnetic material, insulator, ceramics, solid-state crystal, amorphous, glass, liquid, melt, environmental material |
| C. Technique | inelastic scattering, absorption and its secondary process |
| D. Technique (detail) | X-ray Raman spectroscopy, XAFS |
| E. Particular condition | polarization (linear), polarization (circular), low-T (~ liquid N2), room temperature |
| F. Photon energy | X-ray (4-40 keV) |
| G. Target information | chemical state, chemical bonding, local structure, electronic state, spin/magnetism, phase transition |
Industrial keywords
| level 1---Application area | Semiconductor, storage device, cell (battery), environment |
|---|---|
| level 2---Target | HD,MO, fuel cell, catalysis |
| level 3---Target (detail) | magnetic layer |
| level 4---Obtainable information | local structure, electronic state, valence, chemical state |
| level 5---Technique | XAFS, X-ray diffraction |
Classification
A80.14 magnetic materials, A80.34 catalysis, A80.40 environmental materials, M40.10 XAFS
Body text
State-selective XAFS spectroscopy is a powerful technique to study the chemical and electronic states by selecting the state(valence or spin, etc.) of the specified atom.Using this technique,we can obtain information about bonding states, coordinate environment, and electronic states not only of the target element but also of the specified state in the material under the various conditions. This technique can be utilized to various elements such as 3d transition elements, 4f rare-earth elements, and 5d transition elements because linearly or circularly polarized X-rays in hard X-ray region (5-16 keV) are available at BL39XU of SPring-8. This means that the state-selective XAFS spectroscopy can be applied to material science, catalytic chemistry, and environmental science.
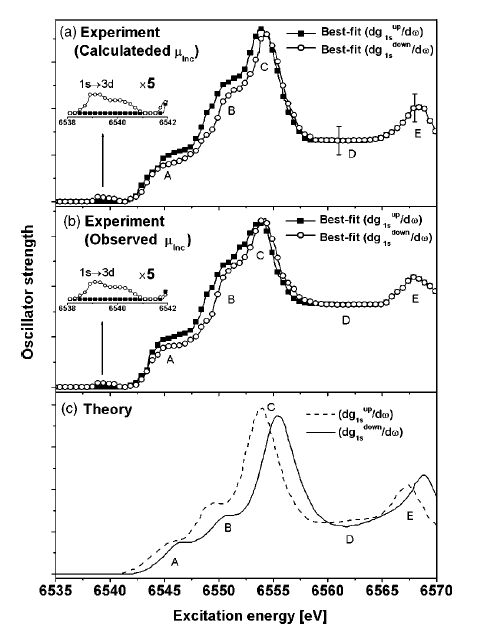
The figure shows spin-selective XAFS spectrum at the Mn K-edge (1s-3d, 4p) of MnO.The XAFS spectrum excited from 1s electrons with up- or down-spin can be obtained individually by analyzing the Mn 3p-1s X-ray emission spectra dependent on the incident X-ray energy.
Fig. Spin-selective XAFS spectrum of MnO derived from Mn 3p-1s X-ray emission spectra.
[ H. Hayashi, M. Kawata, Y. Udagawa, N. Kawamura and S. Nanao, Physical Review B 70, 134427 (2004), Fig. 3,
©2004 American Physical Society ]
Source of the figure
Original paper/Journal article
Journal title
H. Hayashi et al. Phys. Rev. B 70, 134427 (2004).
Figure No.
Fig. 3
Technique
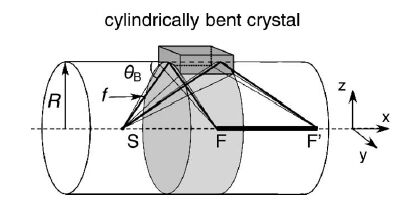
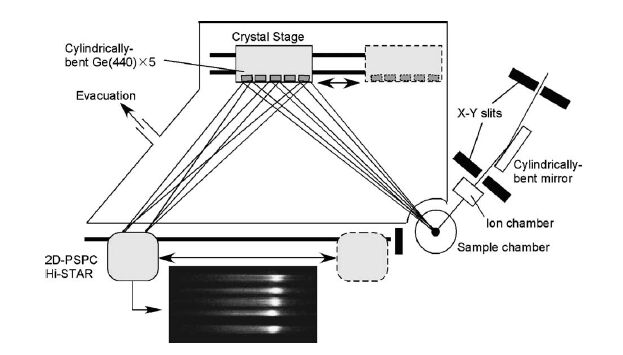
State-selective XAFS spectroscopy is performed by measuring the monochromatized X-ray of fluorescent or inelastic scattered X-rays emitted from the sample as a function of incident X-ray energy. In this example, spin-selective XAFS spectrum were obtained by using the difference of energy loss of excited electrons with between up-spin and down-spin of Mn atoms in MnO. The signal intensity of X-ray emission spectrum is so weak because of a second-order optical process.Recently,a high-sensitive measurement using multi-crystal is performed as shown in Fig. 2.
Fig. 1: Principle of measurement of energy-dispersive (cylindrically focusing) spectrum.
Fig. 2: Multi-crystal type X-ray emission spectrometer (energy-dispersive type).
[ H. Hayashi, M. Kawata, R. Takeda, Y. Udagawa, Y. Watanabe, T. Takano, S. Nanao and N. Kawamura, Journal of Electron Spectroscopy and Related Phenomena 136, 191-197 (2004), Fig. 1, 3,
©2004 Elsevier Science Publisher ]
Source of the figure
Original paper/Journal article
Journal title
H. Hayashi et al., J. Electron Spectrosc. Relat. Phenom. 136, 191 (2004).
Figure No.
Fig. 1, 3
Required time for experimental setup
12 hour(s)
Instruments
| Instrument | Purpose | Performance |
|---|---|---|
| Multi-crystal spectrometer with a two-dimensional position-sensitive detector | measurement of spectrum in wide-energy range of 100-200 eV at a time | energy-dispersive type, 5 crystals |
| X-ray emission spectrometer for magnetic circular dichroism | measurement of magnetic circular dichroism of X-ray emission spectrum | energy-dispersive type, single crystal, maxmum applied magnetic field of 2T |
References
| Document name |
|---|
| H. Hayashi, M. Kawata, Y. Udagawa, N. Kawamura, and S. Nanao, Phys. Rev. B 70, 134427 (2004). |
| H. Hayashi, M. Kawata, R. Takeda, Y. Udagawa, Y. Watanabe, T. Takano, S. Nanao, and N. Kawamura, J. Electron Spectrosc. Relat. Phenom. 136, 191 (2004). |
| 林久史, 分光研究 Vol. 53, No. 5, 283 (2004). |
Related experimental techniques
EXAFS/XAFS spectroscopy, X-ray magnetic circular dichroism, X-ray Raman spectroscopy, Resonant X-ray emission spectroscopy
Questionnaire
The measurement was possible only in SPring-8. Impossible or very difficult in other facilities.
With user's own instruments.
Ease of measurement
Middle
Ease of analysis
Middle
How many shifts were needed for taking whole data in the figure?
Four-nine shifts