Structure determination of Ca-ATPase from rat sarcoplasmic reticulum
Inquiry number
SOL-0000001097
Beamline
BL41XU (Macromolecular Crystallography I)
Scientific keywords
| A. Sample category | biology, medicine |
|---|---|
| B. Sample category (detail) | biomolecule, crystal, protein |
| C. Technique | X-ray diffraction |
| D. Technique (detail) | single crystal |
| E. Particular condition | low-T (~ liquid N2) |
| F. Photon energy | X-ray (4-40 keV) |
| G. Target information | chemical state, chemical bonding, molecular structure, structure analysis, structural change, function and structure, charge density |
Industrial keywords
| level 1---Application area | Pharmaceuticals |
|---|---|
| level 2---Target | drug design, process analytical technology (PAT) |
| level 3---Target (detail) | protein, drug |
| level 4---Obtainable information | crystal structure, local structure |
| level 5---Technique | diffraction, X-ray diffraction |
Classification
M10.10 single crystal diffraction
Body text
Sarcoplasmic reticulum (SR)is the organella of muscle cell, and stores calcium (Ca)ion in it. When the muscle cell is stimulated by neuron, calcium ion is released from SR, and it becomes the trigger of muscle contraction. The calcium ATPase is a membrane protein existing within the SR membrane. It transports calcium ion into SR after muscle contraction.
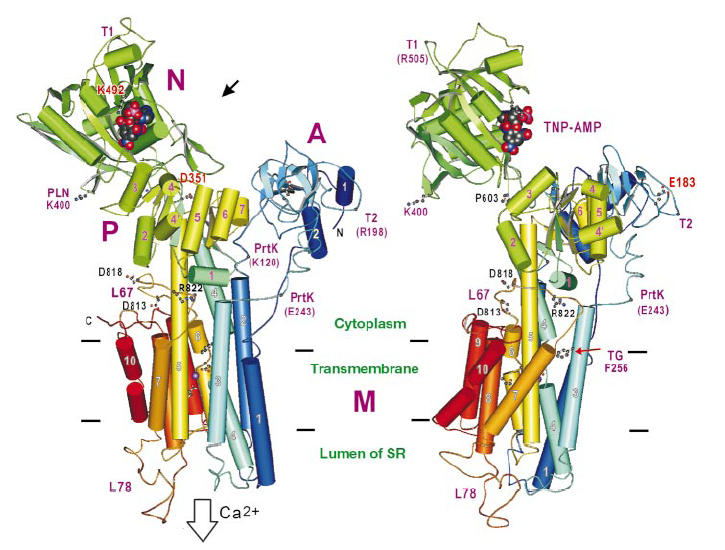
In this case, three-dimensional structure of Ca-ATPase from rat SR was determined by X-ray diffraction experiment at BL41XU (Fig, 1).
Generally, most crystals of membrane protein have only weak ability to diffract X-ray. This enzyme is a large protein with about 110,000 in molecular weight, and the unit cell of its crystal is also large, 166.0x64.4x147.1 Å . In addition, the thickness of its crystal is only less than 20 µm. High brilliant X-ray from undulator of BL41XU was indispensable to collect the good X-ray diffraction data.
Ca-ATPase is paid attention from the viewpoint of cancer treatment and myocardial infarction. Therefore, this result makes those researches advanced greatly.
Figure 1. Structure model of Ca-ATPase
[ C. Toyoshima, M. Nakasako, H. Nomura and H. Ogawa, Nature 405, 647-655 (2000), Fig. 2,
©2000 Nature Publishing Group ]
Source of the figure
Original paper/Journal article
Journal title
C. Toyoshima, et al., Nature 405, 647-655 (2000)
Figure No.
2
Technique
Source of the figure
No figure
Required time for experimental setup
0 hour(s)
Instruments
| Instrument | Purpose | Performance |
|---|---|---|
| Protein Crystal Diffractometer | To record diffraction data |
References
| Document name |
|---|
| C. Toyoshima, et al., Nature 405, 647-655 (2000) |
Related experimental techniques
Questionnaire
The measurement was possible only in SPring-8. Impossible or very difficult in other facilities.
This solution is an application of a main instrument of the beamline.
Ease of measurement
Middle
Ease of analysis
Middle
How many shifts were needed for taking whole data in the figure?
Four-nine shifts