Successful Synthesis of Nanosubstances in Nanotubes - Step towards practical application of nanotubes as reactors for producing new nanotechnology materials - (Press Release)
- Release Date
- 31 Jul, 2008
- BL25SU (Soft X-ray Spectroscopy of Solid)
Scientists at Nagoya University (Shin-ichi Hirano, President) succeeded in producing a new nanowire in collaboration with the Japan Synchrotron Radiation Research Institute (JASRI) (Akira Kira, Director General) and other research institutions by an entirely new method using carbon nanotubes as reactors for synthesizing nanosubstances.
The containment of substances in a carbon nanotube has been previously reported; however, in this study, the scientists succeeded in realizing a new nanoscale synthesis method, in which chemical reactions were induced in a nanotube to transform raw materials into new and different substances. The breakthroughs in this study are the use of carbon nanotubes as reactors focusing on their excellent thermal and chemical resistances and demonstrating the practicality of the nanoscale-controlled synthesis of materials.
The scientists succeeded in synthesizing an approximately 2 nm thick compound nanowire that consists of erbium chloride (ErCl3) molecules orderly arranged in a nanotube. Erbium atoms are covalently bonded to adjacent Cl atoms; the nanowire has a metal complex structure with aligned Er atoms. It is impossible to synthesize nanosubstances with such a structure by conventional methods. They carried out an ultrahigh-sensitivity spectrometric measurement using 1400 eV high-brilliance soft X rays at SPring-8, an advanced measurement technology, to determine whether the synthesized nanosubstances have the designed structure and functions. It was verified that the chemical state of Er ions in the nanowire is Er3+, the same as in ErCl3. This result confirmed the successful synthesis of the substance as designed.
This new nanoscale-controlled synthesis method will enable not only the production of metal complex nanowires but also the development of nanosized resistors and wiring for various devices. This will expand the applications of nanowires to the development of new functional materials. Hence, this new method is a significant achievement, promoting and accelerating the research and development of highly functional nanowire devices using nanotechnology.
This achievement is the result of joint research by Hisanori Shinohara and Ryo Kitaura, professor and research associate of Nagoya University, respectively, Tetsuya Nakamura, senior scientist of JASRI, Tsuyoshi Saito, team leader of Advanced Industrial Science and Technology, and their colleagues. It was reported in Nano Research online on July 31, 2008, and in the August issue of the journal.
Publication:
"High yield synthesis and characterization of the structural and magnetic properties of crystalline ErCl3 nanowires in single-walled carbon nanotube templates"
Ryo Kitaura, Daisuke Ogawa, keita Kobayashi, Takeshi Saito, Satoshi Ohshima, Tetsuya Nakamura, Hirofumi Yoshikawa, Kunio Awaga and Hisanori Shinohara
Nano Research, published online 31 July 2008
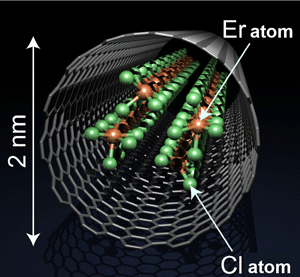 Fig.1 Schematic view of the synthesized ErCl3 nanowire
Fig.1 Schematic view of the synthesized ErCl3 nanowire
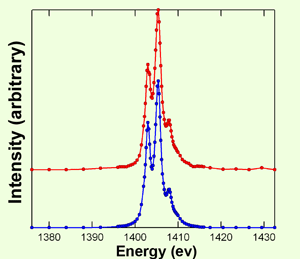 Fig.2 Soft X-ray absorption spectra measured at BL25SU
Fig.2 Soft X-ray absorption spectra measured at BL25SU red: Er2O3 blue: ErCl3 in a nanotube
|
For more information, please contact Prof. Hisanori Shinohara (Nagoya University) |
- Previous Article
- Clarification of Structure of Influenza Virus RNA Polymerase - Possibility of Design of New Drugs against New Influenza Virus (Press Release)
- Current article
- Successful Synthesis of Nanosubstances in Nanotubes - Step towards practical application of nanotubes as reactors for producing new nanotechnology materials - (Press Release)
 .
.