Clarification of Structure of Influenza Virus RNA Polymerase - Possibility of Design of New Drugs against New Influenza Virus (Press Release)
- Release Date
- 28 Jul, 2008
- BL41XU (Structural Biology I)
Key research achievements
- Clarification of binding structure between subunits by X-ray crystal structure analysis
- Identification of amino acid residue that is important for subunit binding
-
Confirmation of deterioration of polymerase activity by inhibition of subunit binding caused by amino variation
- Possibility of development of antiviral drugs for new viruses by designing inhibitor agents
Eiji Obayashi, special researcher associate, and Sam-yong Park, associate professor, of the International Graduate School of Arts and Sciences, Yokohama City University, clarified the structure between the subunits of RNA polymerase, which plays a primary role in replicating influenza virus, at the atomic level for the first time in the world. This was the achievement of joint research with Kyosuke Nagata , professor of the Institute of Basic Medical Sciences, University of Tsukuba and other researchers.
Influenza is caused by viral infection and is well known in Japan as a disease that becomes epidemic every winter. In recent years, there has been concern about a pandemic of the infection of humans by avian influenza virus. The worldwide development of new vaccines and drugs against such a new virus is being carried out aggressively. RNA polymerase of the influenza virus is attracting attention as the target of new drugs because it plays a primary role in replication (proliferation) of the virus.
The joint research group focused on the fact that the three subunits of the RNA polymerase of the influenza virus lose their function when any of them is lacking and revealed the binding structure between the two subunits at the atomic level using SPring-8 and another facility. From the information about the structure, the researchers identified the amino acid residue that is important for the binding of the two subunits. Then, they confirmed that the variation of this amino acid residue inhibits the subunit binding and significantly decreases polymerase activity.
This research achievement has enabled the design of new drugs to inhibit the binding of the two subunits and directly suppress the proliferation of the virus. Moreover, the same interaction between the subunits is found in the RNA polymerase of many types of influenza virus ever discovered including avian influenza; we thus hope that, unlike conventional vaccines, the new drugs developed considering this binding structure will be epoch-making because of their effectiveness for any type of influenza virus.
This research achievement was published in the online version of the British scientific journal Nature (July 27, 2008) and will also be published in the printed version of Nature.
Publication:
"The structural basis for an essential subunit interaction in influenza virus RNA polymerase"
Eiji Obayashi, Hisashi Yoshida, Fumihiro Kawai, Naoya Shibayama, Atsushi Kawaguchi, Kyosuke Nagata, Jeremy R. H. Tame & Sam-Yong Park
Nature advance online publication, published online 27 July 2008
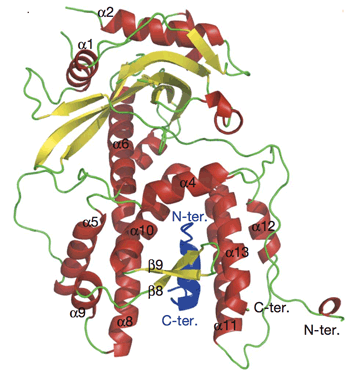 Crystal structure of two bound subunits, PA and PB1, of influenza virus RNA polymerase
Crystal structure of two bound subunits, PA and PB1, of influenza virus RNA polymerase
|
For more information, please contact: |
- Current article
- Clarification of Structure of Influenza Virus RNA Polymerase - Possibility of Design of New Drugs against New Influenza Virus (Press Release)