Observation of Unusual Electron Orbit Using Synchrotron Radiation X-rays (Press Release)
- Release Date
- 06 Mar, 2009
- BL19LXU (RIKEN SR Physics)
- BL29XU (RIKEN Coherent X-ray Optics)
The University of Tokyo
RIKEN
Researchers at the University of Tokyo (Hiroshi Komiyama, President) and RIKEN (Ryoji Noyori, President) were the first in the world to successfully observe electrons of an iridium composite oxide traveling in an unusual orbit using X-ray diffraction.
In the case of a free, isolated atom, electrons orbit its nucleus. On the other hand, the outermost electrons in an atom of a solid are generally immobilized in the presence of adjacent atoms and undergo negligible orbital motion. However, it has been pointed out that, even in a solid, the electrons of an atom with a large atomic number can achieve some orbital motion. The researchers of the joint team succeeded in observing the electron orbit of iridium (Ir; atomic number = 77) in an Ir oxide (Sr2IrO4) by a new method using synchrotron radiation at SPring-8. The orbital direction of the electrons observed in this research corresponds to the direction of the electron spin (similar to the rotation of the earth); this correspondence differs from that in the case of an isolated atom. The researchers also clarified that two types of Ir atom are aligned alternately – one type has electrons with a clockwise spin and orbit and the other type has electrons with a counterclockwise spin and orbit. This finding helped explain the low conductivity (insulating property) of Ir oxides. In short, electrons orbiting an atomic nucleus have difficulty in jumping to adjacent atoms, resulting in a nonconductive substance.
The strong spin-orbital interaction of electrons observed in this research can be effectively used to control the spin, i.e., magnetic information, by electrical and optical methods and to read out the information. We thus hope that this achievement will herald the beginning of the development of novel applications, for example, new magnetic recording and computing devices. This achievement was reported in the American scientific journal Science on 5 March 2009.
Reporters:
Kim Beomjoon (researcher of Graduate School of Frontier Sciences, The University of Tokyo; researcher of RIKEN Advanced Science Institute)
Hidenori Takagi (professor of Graduate School of Frontier Sciences, The University of Tokyo; group director of RIKEN Advanced Science Institute)
Hiroyuki Ohsumi (researcher of RIKEN SPring-8 Center)
Takahisa Arima (team leader of RIKEN SPring-8 Center; professor of Tohoku University)
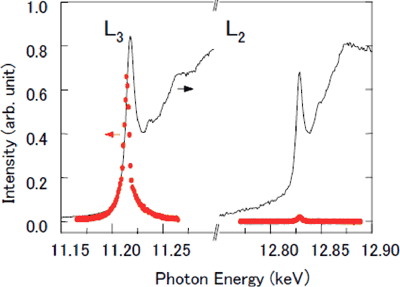 Fig. 1 Energy dependence of magnetic X-ray diffraction intensity (red) and X-ray adsorption intensity (solid black line) for Sr2IrO4.
Fig. 1 Energy dependence of magnetic X-ray diffraction intensity (red) and X-ray adsorption intensity (solid black line) for Sr2IrO4.
An increase in the X-ray adsorption intensity corresponds to the transfer of electrons, whereby resonance enhancement of the diffraction intensity is induced. A marked increase in diffraction intensity is observed at the L3-edge(2P3/2->5d), whereas hardly any increase occurs at the L2-edge (2P1/2->5d). The abscissa indicates the wavelength of X-rays converted to photon energy. The relationship between the two is roughly expressed as
wavelength (nm) × photon energy (keV) = 1.24.
A nanometer is one-billionth of a meter.
Publication:
"Phase-Sensitive Observation of a Spin-Orbital Mott State in Sr2IrO4"
B. J. Kim, H. Ohsumi, T. Komesu, S. Sakai, T. Morita, H. Takagi, and T. Arima
Science Vol. 323. no. 5919, pp. 1329 - 1332 (2009), published online 6 March 2009
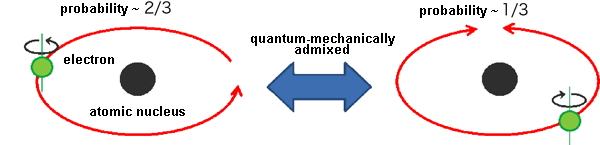 Fig. 2 Schematic illustrating the outermost electrons of the Ir atom revealed in this research.
Fig. 2 Schematic illustrating the outermost electrons of the Ir atom revealed in this research.About two-thirds of the electrons orbited in the same direction as their spin. The other electrons, in contrast, did not orbit because of the opposite direction of the spin. Adjacent Ir atoms have opposite directions of both the spin and orbit.
|
For more information, please contact: Prof. Takahisa Arima (RIKEN) or Dr. Hiroyuki Ohsumi (RIKEN) |
- Previous Article
- Success in Observation of Transverse Acoustic Wave in Liquid! - Can We Overturn the Conventional Textbook Theory? - (Press Release)
- Current article
- Observation of Unusual Electron Orbit Using Synchrotron Radiation X-rays (Press Release)
 ,
, ,
, .
.