Basket protein molecules of 12 nm diameter: Clarification of mechanism behind accumulation of metal ions by ferritin at atomic level -Development of nanobiotechnology using protein molecules (Press Release)
- Release Date
- 18 Mar, 2009
- BL41XU (Structural Biology I)
Kyoto University
Nagoya University
RIKEN
Japan Synchrotron Radiation Research Institute
Key research achievements
- First-ever clarification of the process of accumulation of metal ions in basket protein molecules by single-crystal X-ray structural analysis
- Progress toward clarifying the reaction involved in the formation of bones and pearls
- Hopes for application to the development of semiconductors and magnetic materials
Researchers at Kyoto University (Hiroshi Matsumoto, President), Nagoya University (Shin-ichi Hirano, President), RIKEN (Ryoji Noyori, President), and Japan Synchrotron Radiation Research Institute (Akira Kira, Director General) succeeded in visualizing the process of the accumulation of metal ions in the inner space of ferritin, which is a basket protein molecule that accumulates iron in the body, by single-crystal X-ray structural analysis. This result is expected to lead to not only the clarification of the initial reaction involved in the formation of bones and pearls but also the development of semiconductors and magnetic materials.
The joint research group including Takafumi Ueno, an associate professor and a member of the Kitagawa group at the Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, and Yoshihito Watanabe, a professor of Research Center for Materials Science, Nagoya University, formed crystals of a ferritin complex containing metal ions. They clarified the structural changes of proteins during the accumulation of metal ions inside protein molecules using Structural Biology I Beamline (BL41XU) at SPring-8 in cooperation with the group of Masaki Takada, the chief scientist at RIKEN SPring-8 Center (Harima Institute), and a group from Japan Synchrotron Radiation Research Institute.
Researchers carried out the structural analysis of ferritin crystals after the accumulation of different amounts of metal ions and successfully obtained snapshots of the structural changes inside the basket protein molecules upon the intake of metal ions. It was found that when metal ions are absorbed by basket protein molecules, the side chains of amino acid molecules sequentially bind with many metal ions while changing their structure in the same way that a human carries many large balls with two outstretched arms. This was the first experimental evidence of structural changes in the residues of amino acids during the accumulation of metal ions inside protein molecules. This result provides not only basic knowledge that will help clarify the reaction involved in the formation of bones and pearls but also interesting hints for the development of semiconductors using proteins and new inorganic materials, such as magnetic materials, which have been attracting worldwide attention recently.
The study was supported by Precursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Agency (JST) under the research theme of “Structural Control and Function,” and SPring-8. The research result will be published online in Journal of the American Chemical Society, one of the most influential journals in the field of chemistry in the world, towards the end of March 2009, and will soon be published in the printed version.
Publication:
"Process of Accumulation of Metal Ions on the Interior Surface of apo-Ferritin: Crystal Structures of a Series of apo-Ferritins Containing Variable Quantities of Pd(II) Ions"
Takafumi Ueno, Mizue Abe, Kunio Hirata, Satoshi Abe, Masako Suzuki, Nobutaka Shimizu, Masaki Yamamoto, Masaki Takata and Yoshihito Watanabe
Journal of the American Chemical Society, published online March 24, 2009
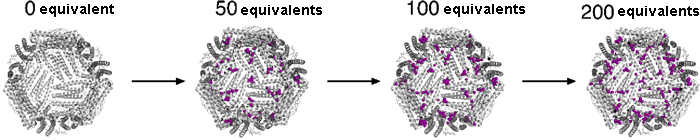 Fig. 1 Crystalline structure of ferritin before reaction with palladium and after reaction with 50, 100, and 200 equivalents of palladium.
Fig. 1 Crystalline structure of ferritin before reaction with palladium and after reaction with 50, 100, and 200 equivalents of palladium.Using the SPring-8 BL41XU beamline, the identification of only palladium atoms is possible. As shown in purple, an increase in the number of palladium atoms bound to the basket was observed.
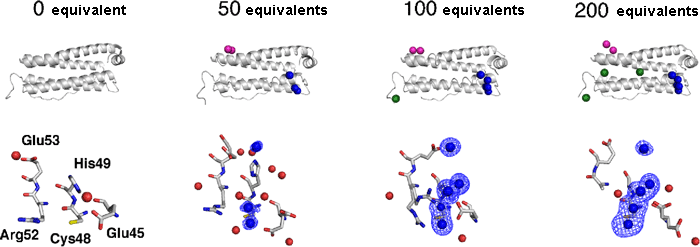 Fig. 2 Details of structural changes with the accumulation of palladium.
Fig. 2 Details of structural changes with the accumulation of palladium. The basket structure of ferritin is composed of 24 proteins, one of which is shown in the figure. The accumulated palladium atoms bind at the sites colored in pink (three-fold axis channel) and blue (metal accumulation sites). Subsequently, the number of metal atoms bound to the metal accumulation site increases; when these sites are occupied by metals with a multiatom structure, the palladium atoms then bind at the sites colored in green (upper). During this process, the structures of the His49 and Glu53 side chains significantly change at the metal accumulation sites with increasing number of bonds (lower). The blue shaded area indicates the electron density of palladium, which can be distinguished using the SPring-8 BL41XU beamline.
|
For more information, please contact: or Prof. Yoshihito Watanabe (Nagoya University) |
- Previous Article
- Observation of Unusual Electron Orbit Using Synchrotron Radiation X-rays (Press Release)
- Current article
- Basket protein molecules of 12 nm diameter: Clarification of mechanism behind accumulation of metal ions by ferritin at atomic level -Development of nanobiotechnology using protein molecules (Press Release)
 ,
, .
.