Flexible Structure of Prostaglandin D Synthase (PGDS) with Various Functions Supporting Sleep Induction, and Maturation and Maintenance of Reproductive System (Press Release)
- Release Date
- 10 Jul, 2009
- BL45XU (RIKEN Structural Biology I)
Flexible change in pouchlike structure enables expression of various functions such as synthesis of sleep-inducing substance.
RIKEN
Osaka Bioscience Institute
Key research achievements
• Discovery of the PGDS pouchlike structure with a lid for opening/closing the structure
• Possibility of inducing brain protection function by combining the flexibility of changing the PGDS structure with the features of substances responsible for Alzheimer's disease
• Relationships between PGDS and diseases, such as obesity due to overeating and arteriosclerosis, remain to be clarified in future research
|
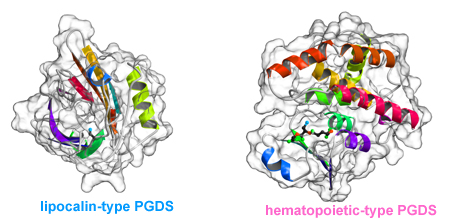
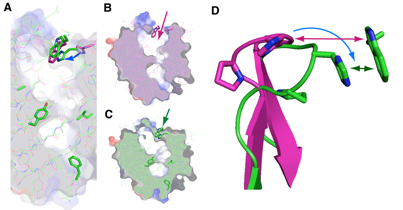
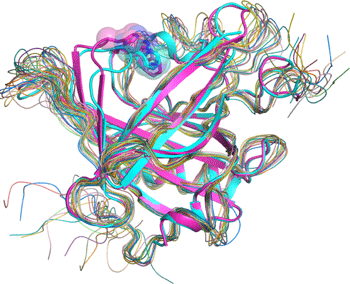
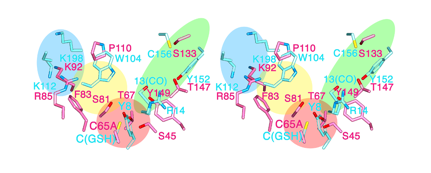
A research group consisting of scientists from RIKEN (Ryoji Noyori, President) and Osaka Bioscience Institute (Osamu Hayaishi, Chairman of the Board of Trustees) determined the two different steric structures of lipocalin-type prostaglandin D synthase (lipocalin-type PGDS)*1 that catalyzes the synthesis of a sleep-inducing substance. It was clarified that the synthase has a pouchlike structure with a lid for opening/closing the structure; by flexibly changing its structure, lipocalin-type PGDS supports vital activities, such as sleeping, and maturation. This was accomplished by a joint research team consisting of Masashi Miyano, chief scientist, and Hideo Ago, senior research scientist, of the Structural Biophysics Laboratory, RIKEN SPring-8 Center, and Yoshihiro Urade, head, and Kousuke Aritake, technician, of the Osaka Bioscience Institute. Lipocalin family proteins*2 hold physiological substances inside a pouchlike structure. Among them, only PGDS has enzyme activity and catalyzes the synthesis of prostaglandin D2 (PGD2), which naturally induces non-rapid-eye-movement (non-REM) sleep.*3 Two types of PGDS, namely, hematopoietic and lipocalin, are known. Hematopoietic-type PGDS is considered to be associated mainly with immunological and irritation controls. Hematopoietic-type PGDS was discovered in 1997, and the research group clarified its crystalline structure and reaction mechanism around the same time. It was found that hematopoietic-type PGDS is related to myonecrosis, and the research and development of it as a possible drug for treating Duchenne muscular dystrophy*4 are under way. Another type of PGDS, i.e., lipocalin-type PGDS, is a small protein composed of only 150 amino acids. It functions in various activities, such as sleeping, and maturation, thus supporting our lives. Although the gene sequence of the protein was identified relatively early, it is difficult to produce large quantities of the protein in its active form because its early-stage recombinant form is unstable. In this study, two types of lipocalin-type PGDS crystals were analyzed using RIKEN Structural Biology I beamline BL45XU at SPring-8, and the scientists involved succeeded in the determination of their steric structures. The result indicates that lipocalin-type PGDS has a pouchlike structure with a lid composed of proteins. Furthermore, taking into account the past reports on the structure of lipocalin-type PGDS such as the results of nuclear magnetic resonance (NMR) analysis*5 conducted by Osaka Bioscience Institute in cooperation with other institutions, they concluded that lipocalin-type PGDS has a very flexible structure. Lipocalin-type PGDS is considered to not only serve as an enzyme protein that catalyzes the synthesis of PGD2, but also serve as a protein that strongly binds to retinoic acid (a derivative of vitamin A), thyroxin hormone, complex sugars, and even β amyloid (a causative substance of Alzheimer's disease) owing to its flexible structure. Their achievement is expected to accelerate the clarification of the brain protection mechanism and the development of drugs for arteriosclerosis. The achievement will be published in the Journal of Biological Chemistry in the US on August 14, 2009. Publication: |
<Figure>
|
|
||||||||||
|
|
||||||||||
<Glossary>
*1 Lipocalin-type prostaglandin D synthase (lipocalin-type PGDS)
Lipocalin-type PGDS belongs to the lipocalin family of proteins, and is the only protein in the family that has enzyme activity.
*2 Lipocalin family proteins
Lipocalin family proteins are capable of holding high-fat physiological substances, such as fatty acids and retinoic acid, in their pouchlike structure, and thus carrying these substances.
*3 Non-rapid-eye-movement (non-REM) sleep
There are two stages of sleep; one is rapid-eye-movement (REM) sleep during which people dream, and the other is non-REM sleep during which the brain is at rest. Non-REM sleep and REM sleep occur alternately. It is known that PGD2, which is synthesized by lipocalin-type PGDS abundant in encephalic liquid, induces natural non-REM sleep.
*4 Duchenne muscular dystrophy
Muscular dystrophy is an inherited progressive muscular atrophy. Duchenne muscular dystrophy is the most common but the symptom is the severest among all types of muscular dystrophy.
*5 Nuclear magnetic resonance (NMR) analysis
NMR analysis is one of the methods of examining molecules and atoms. Approximately 20% of the steric structures of proteins are determined by NMR analysis. Information on dynamic structure or relatively weak binding of molecules of proteins in the liquid state is obtained.
|
For more information, please contact: or |
- Current article
- Flexible Structure of Prostaglandin D Synthase (PGDS) with Various Functions Supporting Sleep Induction, and Maturation and Maintenance of Reproductive System (Press Release)
 ,
, .
.