Clarifying New Phase of Water - One Step Closer to Clarification of Mysterious Properties of Water (Press Release)
- Release Date
- 29 Mar, 2010
- BL04B1 (High Temperature and High Pressure Research)
- BL14B1 (JAEA Materials Science)
Japan Atomic Energy Agency
|
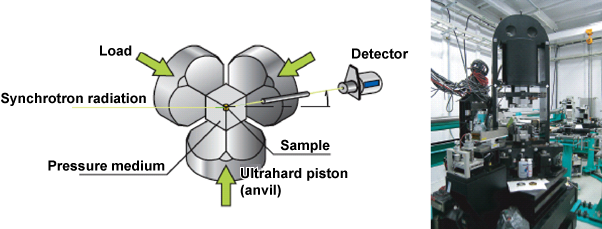
Takashi Ikeda, an associate senior scientist, and Yoshinori Katayama, a senior scientist, at Quantum Beam Science Directorate, Japan Atomic Energy Agency (Toshio Okazaki, President) and their colleagues have successfully determined the structure of water under high pressure and clarified a new phase of water, different from its usual phase. This work was achieved by first-principles molecular dynamics calculation*1 using a supercomputer and by synchrotron radiation X-ray diffraction experiment at SPring-8. Water is the liquid most familiar to us, but is an unusual liquid, which exhibits a property different from that of other liquids. For example, conventionally, liquids expand with increasing temperature, whereas water shrinks as temperature increases from 0 °C to about 4 °C. Also, the boiling temperature of water is much higher than those of liquids comprising other molecules of similar molecular weights. This is considered to be because hydrogen bonds*2 are formed between adjacent water molecules and an orderly state similar to ice, a solid state of water, remains in the molecular arrangement of the water in the liquid state. However, how such a special arrangement changes at higher temperatures has been veiled in mystery because a high pressure sufficient for maintaining the density of water at a constant value is required to unravel the mystery. In this study, the research team has clarified, by theoretical calculation, that no stable hydrogen bonds are formed in water under high pressure and temperature, even when the density of this water is the same as that of water under normal pressure and temperature, because the molecules of this water undergo reorientation at an extremely high speed. The team has also clarified that the molecular arrangement becomes similar to that of usual liquids. In addition, the team has succeeded in an X-ray diffraction experiment by realizing a high temperature of at least 400 °C while maintaining the density of water at that of water under normal pressure and temperature upon the application of a pressure of nearly 1 GPa at SPring-8, and confirmed the above arrangement. We hope that these achievements will help us clarify the importance of the role of water in the degradation and synthesis of materials in the earth's interior under high pressure and temperature. This work was supported by Grants-in-Aid for Scientific Research on Innovative Areas "Earth Science Based on the High Pressure and Temperature Neutron Experiments." The results of this study were published online in an American scientific journal, Journal of Chemical Physics, on 26 March 2010. Publication: |
<Figure>
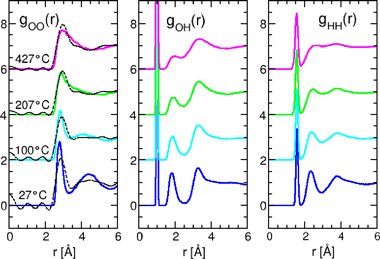
The solid and dotted lines represent the radius distribution functions obtained by calculation and experiment, respectively. The X-ray diffraction experiment yields only gOO(r). For easy distinction, the data for different temperatures are each shifted by two along the ordinate.
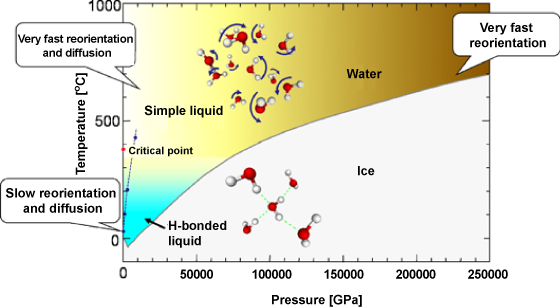
Characteristic structures of water under high pressure and temperature and of that under normal pressure and temperature are shown in the upper and lower parts of the figure, respectively. The red and white balls represent the oxygen and hydrogen atoms, respectively. The hydrogen bond is represented by the green dotted line. The blue dotted line represents the relationship between temperature and pressure when the density of water is 1.0 g/cm3. Calculation was carried out under the conditions shown by blue circles.
<Glossary>
*1 First-principles molecular dynamics calculation
First-principles molecular dynamics calculation is a simulation method for calculating the force applied to each atom while accurately considering the electron state of a system comprising a number of atoms without using experiential parameters to obtain the temporal development of the locus for each atom. This method enables the theoretical determination of the state of any material under arbitrary thermodynamic conditions. For liquids, however, it is necessary to use a supercomputer because huge calculation is required.
*2 Hydrogen bond
Hydrogen atoms covalently bonded to electron-attracting atoms, such as oxygen and nitrogen, are weakly positively charged because the electrons of the hydrogen atoms are drawn to such atoms, causing a weak bond with the adjacent negatively charged atoms at a strength one-tenth that of the covalent bond. This is referred to as the hydrogen bond. The hydrogen bond formed in water molecules is a relatively strong intermolecular bond.
For more information, please contact: |
- Previous Article
- Dr. Masato Kotsugi Takes Top Place in Japan Institute of Metals Micrograph Award (Topic)
- Current article
- Clarifying New Phase of Water - One Step Closer to Clarification of Mysterious Properties of Water (Press Release)
