Clarifying the Reason for the Strength of a Polymer Battery Material - Hopes for a new battery material (Press Release)
- Release Date
- 03 Feb, 2011
- BL02B2 (Powder Diffraction)
Japan Synchrotron Radiation Research Institute
RIKEN
University of Tsukuba
Shimane University
|
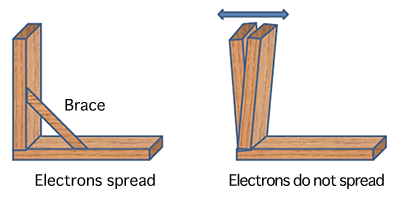
Using high-brilliance X-rays at SPring-8, scientists from Japan Synchrotron Radiation Research Institute (JASRI; President, Tetsuhisa Shirakawa), RIKEN (President, Ryoji Noyori), University of Tsukuba (President, Nobuhiro Yamada), and Shimane University (President, Hiroki Yamamoto) have clarified, for the first time in the world, why the network structure of a cathode material used for lithium-ion polymer batteries is resistant to changes caused by the movement of lithium ions from the negative electrode to the positive electrode and back. Lithium-ion batteries, which are expected to be applied as the power source of electric vehicles, are discharged and charged by the movement of lithium ions from the negative electrode to the positive electrode and back. Lithium-ion polymer batteries are expected to be used as low-cost, long-lifetime batteries that can store a large quantity of electricity, and their materials are considered to form a network similar to a jungle gym, with lithium ions stored in the spaces of the network. However, the binding states of lithium ions with iron and cobalt ions in batteries during charging and discharging have remained unclear, which has caused a bottleneck in the development of new battery materials. In this research, using the high-brilliance X-rays at SPring-8, it was found that electrons involved in binding in a material used for lithium-ion polymer batteries move among the constituents of its network, such as iron and cobalt ions and carbon atoms, and spread throughout the polymer in a state corresponding to charging or discharging. These spreading electrons act as a "brace," as seen in wooden buildings, making the network structure resistant to changes caused by the movement of lithium ions. Therefore, the quantity of stored electricity and the durability of lithium-ion polymer batteries may be increased by replacing iron ions with ions of a more suitable metal. Materials used for lithium-ion polymer batteries have been attracting attention as new battery materials with both flexibility and strength, which cannot be achieved with conventional oxide battery materials.* This research group aims to realize highly durable lithium-ion polymer batteries that can store a large quantity of electricity, through future research and development using high-brilliance X-rays. This research was carried out by Yutaka Moritomo (professor of University of Tsukuba, visiting scientist of JASRI), Jungeun Kim (associate senior scientist of JASRI), Kenichi Kato (scientist of RIKEN), Masaki Takata (chief scientist of RIKEN), and Hiroshi Tanaka (associate professor of Shimane University). The achievements of this research were published online in Applied Physics Express, a journal published by the Japan Society of Applied Physics, on 3 February 2011. (Publication) |
<<Glossary>>
* Oxide battery material
LiCoO2 has been used in practice as an oxide battery material. An electric charge of 140 mA/h can be stored per gram of LiCoO2. Currently, nickel- and manganese-based oxide battery materials are being developed because cobalt is expensive.
<<Figure>>
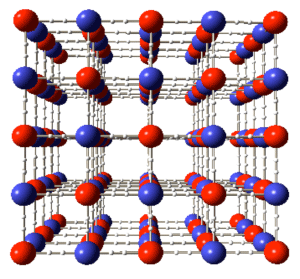
The red and blue spheres represent transition metals, and the bars represent cyano-groups.
used in lithium-ion polymer batteries
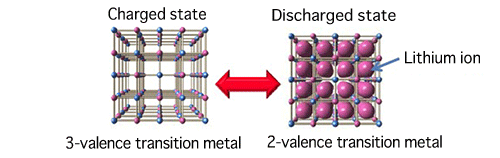
The red and blue spheres represent transition metals, and the larger pink spheres represent lithium ions.
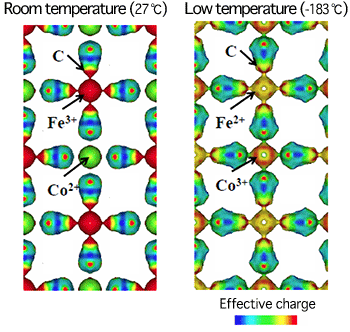
(the number of electrons per 10-24 cm3 is 0.7)
at room temperature (27oC) and at a low temperature (-183oC)
The colors illustrate the degree of effective charges in the inner atoms.
and strength of network structure
|
For more information, please contact: |
- Previous Article
- Clarifying the Mechanism of Transparent Cobalt-Doped Titanium Dioxide Thin Films Exhibiting Magnetism (Press Release)
- Current article
- Clarifying the Reason for the Strength of a Polymer Battery Material - Hopes for a new battery material (Press Release)