Clarification of Structure and Molecular Mechanism of Reductase that Eliminates Misfolded Proteins in Cells (Press Release)
- Release Date
- 19 Feb, 2011
- BL44XU (Macromolecular Assemblies)
Kyoto Sangyo University
Kyushu University
|
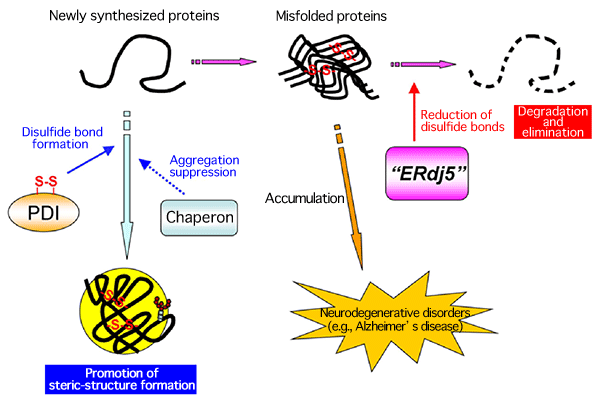
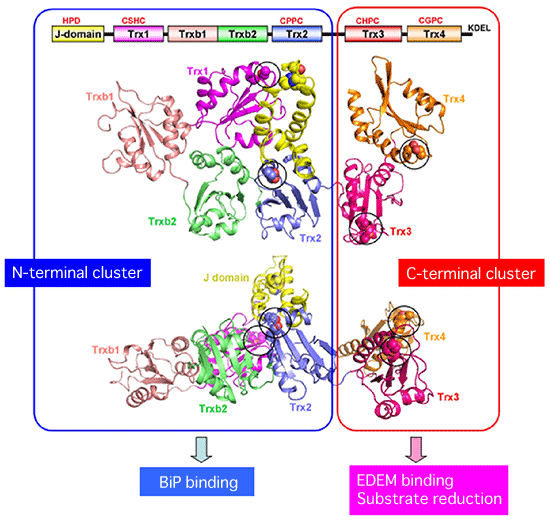
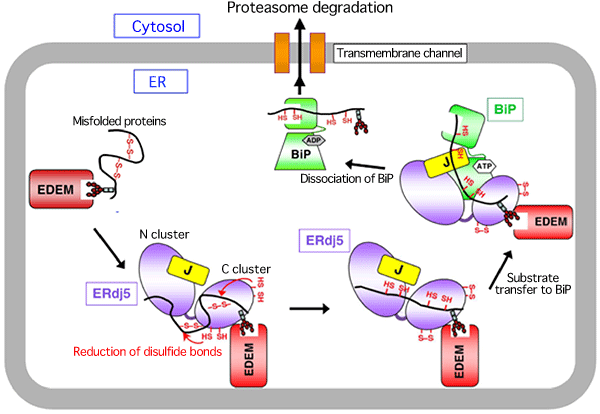
A research group consisting of scientists of Kyoto Sangyo University, Kyushu University, Kyoto University, and Osaka University succeeded in clarifying the crystal structure of a reductase, ERdj5, at a high resolution and its molecular mechanism [Representatives of the group: Kazuhiro Nagata, a professor of the Faculty of Life Sciences of Kyoto Sangyo University, and Kenji Inaba, a research associate professor of the Institute for Advanced Study (Medical Institute of Bioregulation) of Kyushu University]. ERdj5 promotes the degradation and elimination of misfolded proteins by reducing incorrect disulfide bonds generated erroneously in the endoplasmic reticulum (ER) of mammalian cells. The research group determined, for the first time in the world, the crystal structure of full-length ERdj5, which is involved in the degradation of ER proteins in the cells of higher organisms. Owing to these achievements, the mechanism of the quality-control process of proteins in mammalian cells was partially clarified. It is considered that the misfolded proteins accumulated in cells contribute to the development of neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases; it is hoped that the causes of these diseases can be clarified at the molecular level in the future. These research achievements were published online in the American scientific journal Molecular Cell (published by Cell Press) on 18 February 2011. (Publication) |
<<Figure>>
|
For more information, please contact: |
- Previous Article
- Clarifying the Reason for the Strength of a Polymer Battery Material - Hopes for a new battery material (Press Release)
- Current article
- Clarification of Structure and Molecular Mechanism of Reductase that Eliminates Misfolded Proteins in Cells (Press Release)