World’s First Successful Fabrication of Nanotubes in a Flask by Simple Synthesis Method (Press Release)
- Release Date
- 28 Feb, 2011
- BL01B1 (XAFS)
Kyoto University
Japan Science and Technology Agency (JST)
Japan Synchrotron Radiation Research Institute (JASRI)
|
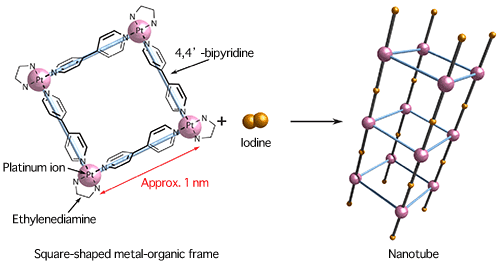
A research group of Kyoto University (President, Hiroshi Matsumoto) has succeeded in fabricating semiconducting nanotubes that can selectively adsorb molecules, in joint research with Japan Synchrotron Radiation Research Institute (JASRI). The joint research group was led by Professor Hiroshi Kitagawa and the research scientist Kazuya Otsubo at Kyoto University. Adsorbents, typified by activated charcoal and zeolites, adsorb molecules and are called porous materials*1 because they contain a large number of small pores. Recently, porous metal-organic complexes with higher selective gas adsorption capability than activated carbon and zeolites have been attracting attention as porous materials exhibiting highly efficient functions for isolating and condensing molecules. Thus, the research and development of porous metal-organic complexes as a third type of porous material has been carried out worldwide. On the other hand, carbon nanotubes*2 are being applied as materials in electron devices because of their high conductivity and durability. Carbon nanotubes are also expected to be used as adsorbents because they contain nanometer-size pores (a nanometer is one-billionth of a meter). However, carbon nanotubes are generally synthesized from their constituent elements at high temperatures (at least 1000oC), making it difficult to control their size and shape. In this study, the research group focused on a bottom-up approach,*3 in which metal-organic frames are assembled from metal ions and organic molecules. They succeeded, for the first time in the world, in synthesizing an assembly of square-pillar nanotubes at room temperature with a diagonal of approximately 1.5 nm and each nanotube having exactly the same shape. These nanotubes can selectively adsorb water and alcohol vapor because they contain nanometer-sized pores. In addition, the nanotubes exhibit semiconductor properties and their various electronic properties can be controlled by exchanging structural components with guest molecules. The above research achievements are expected to lead to the application of porous materials to new sensors and electron devices. This study was carried out as part of research with the theme "Creation of the Metal-Organic Hybrid Protonics and Functional Nano-Layer Integrated Systems" (Research Director: Professor Hiroshi Kitagawa, Kyoto University) in the research field "Development of the Foundation for Nano-Interface Technology" in the Core Research for Evolutional Science and Technology (CREST) project supported by JST. It was also supported by JASRI as a SPring-8 research proposal. The original paper reporting this research was published online in the British scientific journal Nature Materials on 27 February 2011. (Publication) |
<<Glossary>>
*1 Porous materials
Porous materials refer to materials containing a large number of pores. Pores in porous materials are classified according to their diameter as macropores (> 50 nm), mesopores (2-50 nm), and micropores (< 2 nm). In particular, the diameter of micropores is close to that of molecules, meaning that micropores have long been attracting attention for their applicability to the adsorption and isolation (molecular sieving) of various molecules.
*2 Carbon nanotubes
Carbon nanotubes were discovered by Sumio Iijima in 1991. They have a tubular structure with a diameter of 0.7-1.4 nm and have the appearance of a rolled-up honeycomb-like sheet consisting of a hexagonal lattice with each hexagon having six carbon atoms at its vertices (similar to graphite, pencil lead). Carbon nanotubes can be a metal or a semiconductor depending on the way in which the honeycomb-like sheet is rolled up.
*3 Bottom-up approaches
Bottom-up approaches refer to methods of building an entire structure from its fundamental parts, the opposite of top-down approaches. Bottom-up approaches suggest a technique for fabricating fine materials and devices by assembling tens or hundreds of atoms and molecules. Bottom-up approaches are relatively new techniques, and notable examples include the self-assembly method, in which a nanoscale structure is spontaneously assembled, and the sequential lamination method, in which a nanoscale structure is artificially assembled. Bottom-up approaches are effective for processing and manufacturing nanostructures with a size of several tens of nanometers or less. In this study, small building blocks such as metal ions and organic molecules were assembled to form a target nanostructure.
<<Figure>>
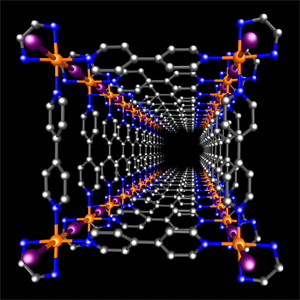
Square-pillar nanotubes are created through the reaction of a cubic metal-organic frame and iodine.
Well-defined nanotubes were assembled by the reaction shown in Fig. 1. In the figure, the orange spheres represent platinum, the purple spheres represent iodine, the blue spheres represent nitrogen, and the gray spheres represent carbon.
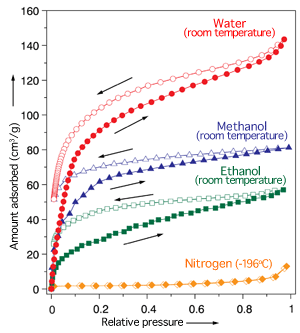
The amounts of water (red), methanol (blue), and ethanol (green) molecules adsorbed into the nanotubes increase with increasing vapor pressure. No nitrogen (orange) is adsorbed.
|
For more information, please contact: |
- Previous Article
- Successful Development of Method Capable of Simply and Quickly Analyzing Characteristics of Membrane Proteins (Press Release)
- Current article
- World’s First Successful Fabrication of Nanotubes in a Flask by Simple Synthesis Method (Press Release)