Determining Crystal Structure of Acetabularia Rhodopsin II, a Difficult-to-Synthesize Membrane Protein (Press Release)
- Release Date
- 29 Jun, 2011
- BL41XU (Structural Biology I)
RIKEN
Key research achievements
o First-ever determination of crystal structure of rhodopsin in unicellular eukaryotes
o Analyses of properties and structures of membrane proteins using the cell-free protein synthesis system
o Potential applications of research results to the analyses of functions and structures of membrane proteins that are industrially useful, for example, those that can be used in the development of medicines
Using RIKEN’s original cell-free protein synthesis system,*1 scientists of RIKEN (Ryoji Noyori, President) have determined, for the first time in the world, the crystal structure of Acetabularia rhodopsin II (ARII), which is a membrane protein and a light-driven proton pumping*2 rhodopsin*3 in unicellular eukaryotes.*4 The achievements of this study were realized by a joint research group led by Shigeyuki Yokoyama (Director), Takashi Wada (Research Scientist), Mikako Shirouzu (Senior Scientist), Masakatsu Hato (Senior Scientist), and Tomomi Someya (Senior Scientist) of RIKEN Systems and Structural Biology Center and Kazumi Shimono (Assistant Professor, also a RIKEN Visiting Scientist) and Naoki Kamo (Professor) of Matsuyama University. ARII is a membrane protein called rhodopsin from a marine alga, Acetabularia acetabulum,*5 and is very difficult to mass-synthesize by conventional methods using living cells. In this research, however, the scientists have succeeded, for the first time in the world, in synthesizing a large quantity of ARII with complete functions using the cell-free protein synthesis system that was originally developed by RIKEN. In studies of proteins, it is essential to obtain intact proteins. The achievements of this study enabled investigation of the functions of ARII, which was found to pump hydrogen ions (protons) from the inside to the outside of cells upon light irradiation, i.e., ARII is a light-driven proton pump. When the lipidic mesophase method,*6 by which membrane proteins are crystallized in an artificial lipid bilayer membrane*7 mimicking a natural cell membrane, was applied to ARII, ARII was successfully crystallized in the lipid bilayer membrane with its structure stably maintained. In addition, SPring-8 X-ray beams were irradiated onto the obtained ARII crystals, and the crystal structure was analyzed and accurately determined at a high resolution*8 of 3.2 Å. This was the first successful structural analysis of rhodopsin from unicellular eukaryotes. The method of synthesizing membrane proteins using the cell-free protein synthesis system, which was adopted in this study, is expected to be widely applied to the analysis of the functions and structures of membrane proteins that are industrially useful, for example, those that can be used in the development of medicines. This research was supported by the Targeted Proteins Research Program, a large-scale research and development project launched in 2007 by the Ministry of Education, Culture, Sports, Science and Technology. The research results were published online in the scientific journal Journal of Molecular Biology on 25 June 2011. Publication: |
<<Glossary>>
*1 Cell-free protein synthesis system
The cell-free protein synthesis system is an artificial system that is independent of living organisms and can be used to synthesize proteins by extracting sets of components necessary for synthesizing proteins from cells and adding genes that encode target proteins and can be read by the system to the extracted components. This system has many advantages: e.g., it is easy to add various external factors and change and optimize reaction conditions. RIKEN is developing this cell-free synthesis system for proteins that are difficult to synthesize, such as membrane proteins, as part of the Targeted Proteins Research Program.
*2 Light-driven proton pumps
Pumps are used to force water to flow from a low position to a high position using energy. In biological systems, membrane proteins that force substances to flow from a low-concentration site to a high-concentration site (Note: this may apply to other features than concentration) using energy are called pumps. Light-driven proton pumps are membrane proteins that force protons (hydrogen ions) to flow using optical energy. Various proton pumps function in the human body.
*3 Rhodopsins
Rhodopsins are membrane proteins that sense the brightness of light and exist in the retina of higher animals. Retinal, a derivative of vitamin A, chemically bonds to proteins. When light is absorbed into retinal, the structure of rhodopsin changes. This change serves as a signal, which is transmitted to the brain to sense the light.
*4 Unicellular eukaryotes
Unicellular eukaryotes are single-celled organisms with a nucleus. They include yeasts, amoebae, foraminifera, and some algae.
*5 Acetabularia acetabulum
Acetabularia acetabulum is a marine alga living in warm oceans. This is a huge unicellular eukaryote and consists of a rhizoid, a stalk, and a pileus. Its gamete swims towards light.
*6 Lipidic mesophase method
This is a new technique for crystallizing membrane proteins in an artificial lipid bilayer membrane, and is suitable for the crystallization of membrane proteins that are unstable outside biological bodies. RIKEN is developing a lipidic mesophase technique for proteins that are difficult to crystallize, such as membrane proteins, as part of the Targeted Proteins Research Program.
*7 Lipid bilayer membrane
The hydrophilic region of lipids, particularly polar lipids such as phospholipids, comes in contact with the water phase in an aqueous solution, and hydrophobic regions are aligned in parallel by hydrophobic bonding, thus forming a bilayer structure. This is considered to be the fundamental structure of biological cell membranes and serves as a barrier to the outside environment of cells. Various membrane proteins are embedded in lipid bilayer membranes and play important roles in living organisms, e.g., signaling and substance transport.
*8 Resolution
The resolution refers to the minimum distance between distinguishable adjacent points on an image. The resolution is in a unit of angstrom (Å; 1 Å = 1×10-10 m). The smaller the value, the higher the resolution, indicating that the structure is observed at a higher accuracy.
<<Figures>>
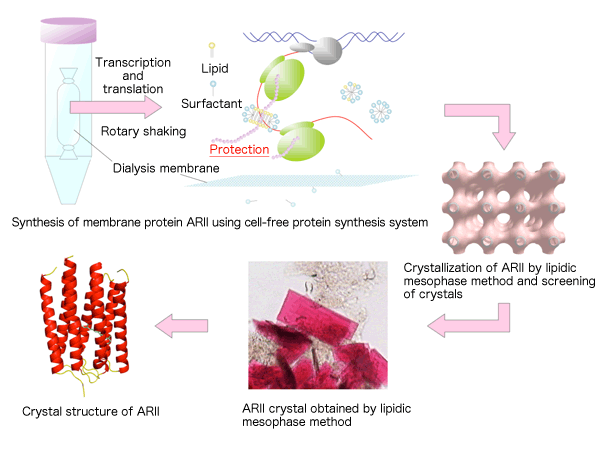
of ARII to analysis of ARII crystal structure
The membrane protein ARII was synthesized using the cell-free protein synthesis system and crystallized by the lipidic mesophase method. X-rays were irradiated onto the obtained crystals, and the crystal structure was analyzed from diffraction images.
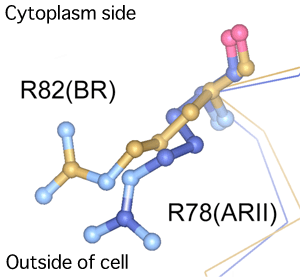
between ARII and bacteriorhodopsin (BR)
For BR, the 82nd arginine residue (R82) usually faces towards the inside of a cell. Upon the irradiation of light, the structure of BR changes to form an M intermediate. At this point, R82 turns towards the outside of the cell and triggers the transport of protons. For ARII, in contrast, the 78th arginine (R78), positioned at the same structural site as that of the R82 in BR, originally faces towards the outside of the cell. This indicates the difference in mechanism behind proton transport between BR and ARII.
|
For more information, please contact: |
- Previous Article
- Discovery of New Materials That Shrink upon Heating (Press Release)
- Current article
- Determining Crystal Structure of Acetabularia Rhodopsin II, a Difficult-to-Synthesize Membrane Protein (Press Release)