Establishment of General Rule for Crystal Structure of Rare-Earth Metal Hydrides (Press Release)
- Release Date
- 05 Jul, 2011
- BL09XU (Nuclear Resonant Scattering)
- BL10XU (High Pressure Research)
Japan Synchrotron Radiation Research Institute
National Institute of Advanced Industrial Science and Technology
Japan Atomic Energy Agency
Osaka University
|
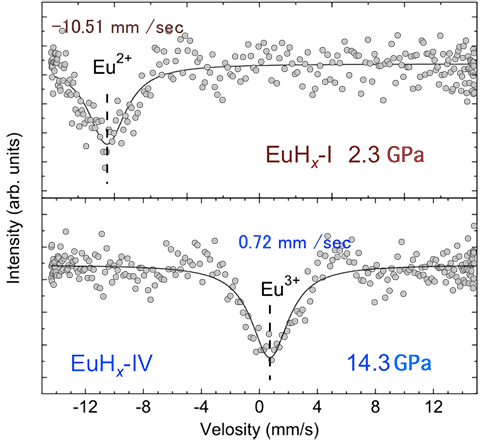
A joint research team of scientists from Japan Synchrotron Radiation Research Institute (JASRI; President, Tetsuhisa Shirakawa), the National Institute of Advanced Industrial Science and Technology (AIST; President, Tamotsu Nomakuchi), Japan Atomic Energy Agency (JAEA; President, Atsuyuki Suzuki), Osaka University (President, Kiyokazu Washida), and the New Energy and Industrial Technology Development Organization (NEDO; Chairman, Seiji Murata) has established a new general rule for the crystal structure of rare-earth metal hydrides, which depends on hydrogen concentration, using high-brilliance X-rays at SPring-8, for the first time in the world. Rare-earth metals*1 have been attracting attention as the constituent elements of high-performance hydrogen storage materials because one metal atom can adsorb up to three hydrogen atoms to form a high-concentration hydride. Solid solutions with a low hydrogen concentration, called α-phase solid solutions, take various crystal structures. In contrast, β-phase hydrides, such as dihydrides (one metal atom adsorbs two hydrogen atoms) and trihydrides (one metal atom adsorbs three hydrogen atoms), both of which have higher hydrogen concentration, take the face-centered cubic (fcc) metal lattice structure. It has been considered that europium (Eu) does not follow the above rule. Therefore, a general rule for the relationship between the hydrogen concentration and crystal structure, which would be indispensable for developing high-performance hydrogen storage materials using rare-earth metals, has not been established so far. The research group observed the crystal structure and valence of Eu hydride exposed to hydrogen at a high pressure exceeding 1 GPa and succeeded in determining their changes by X-ray diffraction and Mössbauer absorption spectroscopy*2 using the synchrotron radiation at SPring-8. It was confirmed that Eu hydride follows the structural rule observed for conventional hydrides; concretely, the presence of trivalent Eu hydride with an fcc metal lattice structure, which has a higher hydrogen concentration than the conventionally known divalent Eu hydride, was confirmed. As a result, a general rule for the crystal structure, which depends on the hydrogen concentration and is applicable to all rare-earth metal hydrides, has been established. This new structural rule indicates the possibility of controlling the crystal structure and hydrogen concentration in the Eu hydride by changing the valence. Control of the valence is expected to be the key to improving the performance of hydrogen storage materials developed using rare-earth metal alloys. The achievement of this study will greatly contribute to the generation of next-generation clean energy, as well as to the development of electronic and magnetic materials utilizing the newly clarified relationship between hydrogen concentration and metal lattice structure because the close relationship between the valence of rare-earth metal atoms and hydrogen concentration was clarified. This achievement was obtained by a group of the following members: Takahiro Matsuoka (Contract Researcher; currently, Specially Appointed Assistant Professor of Osaka University), Naohisa Hirai (Research Scientist), Yasuo Ohishi (Senior Scientist), and Yoshitaka Yoda (Senior Scientist) of JASRI; Hiroshi Fujihisa (Senior Researcher) of AIST; Takaya Mitsui (Associate Chief Scientist), Ryo Masuda (Research Scientist for Specific Project), Akihiko Machida (Research Scientist), Katsutoshi Aoki (Research Scientist for Specific Project), and Makoto Seto (Visiting Scientist; also, Professor at Kyoto University Research Reactor Institute) of JAEA; and Katsuya Shimizu (Professor) of Osaka University. This work was supported by Grants from the NEDO project "Advanced Fundamental Research on Hydrogen Storage Materials" under the title "Experimental Elucidation of Hydrogen-Material Interactions" as a SPring-8 research proposal. The research results were published in the American scientific journal Physical Review Letters on 5 July 2011. Publication: |
<<Glossary>>
*1 Rare-earth metals
Rare-earth metals are the set of 17 elements consisting of 15 elements from lanthanum (La) to lutetium (Lu) in the periodic table and scandium (Sc) and yttrium (Y).
*2 Mössbauer absorption spectroscopy
The Mössbauer effect is a phenomenon in which the resonant absorption of X-rays is induced without the recoil of the nucleus. A Mössbauer absorption spectrum is obtained when gamma (γ) rays emitted from a radioisotope as a radiation source are irradiated on the nucleus of a sample that contains the same species as the source while changing the energy of the γ rays, where the transmitted γ rays are measured using a detector placed behind the sample. When the energy of the γ rays emitted from the source and the excitation energy of the nucleus in the sample are the same, resonant absorption due to the Mössbauer effect is induced in the sample and dips are observed in the spectra. The state of an element in a material including its valence, electronic structure, and magnetism can be determined using the spectra because the energy level of its nucleus is affected by the surrounding electrons. The Mössbauer absorption spectroscopy performed in this study adopted the irradiation of X-rays that were obtained by dispersing high-brilliance synchrotron radiation with a high resolution of nanoelectron volt order. The measurement apparatus was developed as part of the Novel Measuring and Analytical Technology Contributions to the Elucidation and Application of Material Project (Research Supervisor, Michiyoshi Tanaka, Professor Emeritus of Tohoku University) of the Core Research for Evolutional Science and Technology (CREST) Program of Japan Science and Technology Agency (JST) under the title "Research on Nuclear Resonant Scattering Using Synchrotron Radiation for Materials Science" (Representative, Makoto Seto).
<<Figures>>
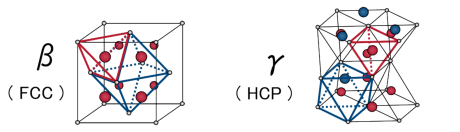
β phase [face-centered cubic (fcc) metal lattice] (left) and γ phase [hexagonal close-packed (hcp) metal lattice] (right). White spheres represent metal atoms, and red and blue spheres in the metal lattices represent hydrogen atoms at the tetrahedral and octahedral sites, respectively.
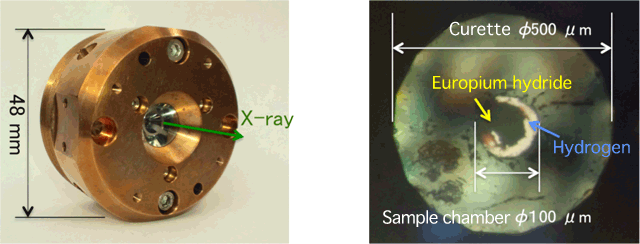
and sample in the apparatus (right)
Curette represents the size of the diamond tip.
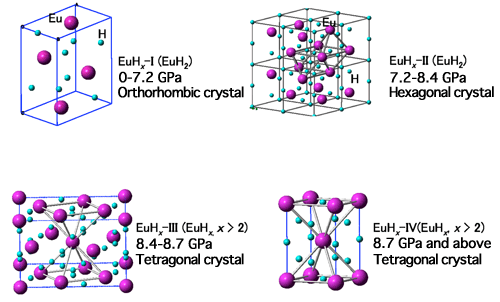
(Purple spheres: Eu atoms; Light-blue spheres: hydrogen atoms)
|
For more information, please contact: Dr. Hiroshi Fujihisa (AIST) Dr. Yasuo Ohishi (JASRI) Dr. Yoshitaka Yoda (JASRI) Dr. Katsutoshi Aoki (JAEA) |
- Previous Article
- Determining Crystal Structure of Acetabularia Rhodopsin II, a Difficult-to-Synthesize Membrane Protein (Press Release)
- Current article
- Establishment of General Rule for Crystal Structure of Rare-Earth Metal Hydrides (Press Release)