Clarifying Three-Dimensional Structure of Adenomatous Polyposis Coli-Sam68 Complex Involved in Development of Colon Cancer (Press Release)
- Release Date
- 12 Oct, 2011
- BL26B2 (RIKEN Structural Genomics II)
RIKEN
Institute of Molecular and Cellular Biosciences, The University of Tokyo
Key research findings
• Clarification of three-dimensional (3D) structure of complex of tumor-suppressing protein adenomatous polyposis coli (APC) and Sam68 by X-ray crystallography
• Identification of mutation of APC causing cancerous changes in cells
• Provision of important knowledge on molecular mechanism of development of colon cancer that will lead to development of treatment strategies
Scientists in RIKEN (Ryoji Noyori, President) succeeded in determining the structure of the functionally important part of adenomatous polyposis coli (APC), which is a protein considered to be involved in the development of colon cancer. They also succeeded in determining the 3D structure of the complex of APC and its binding factor Sam68 (APC-Sam68 complex), obtaining significant findings that will lead to the development of new strategies for the treatment of colon cancer. This was achieved through a joint research by Shigeyuki Yokoyama (Professor of UT-RIKEN Cooperation Laboratory of Structural Biology, Graduate School of Science, The University of Tokyo, also Director of RIKEN Systems and Structural Biology Center), Ella Czarina Morishita (a special research scientist of RIKEN Systems and Structural Biology Center), and Tetsu Akiyama (Professor of Institute of Molecular and Cellular Biosciences and Graduate School of Science, The University of Tokyo). The number of patients with colon cancer is increasing compared with that of patients with other cancers. There are 235,000 patients with colon cancers in Japan according to the Patient Survey conducted by the Ministry of Health, Labour and Welfare in 2008. Moreover, colon cancer is one of the leading causes of death from cancer around the world. Effective treatments have not been found, partly because colon cancer has not been fully clarified at the molecular level. Thus far, the "APC gene" has been identified as an important gene involved in the development of colon cancer. Because mutations in this gene are found in most of the patients with colon cancer, this gene is considered to act as a tumor suppressor gene. It is assumed that APC has a complicated higher-order structure because this protein is very large. Researchers have focused on the domain named the armadillo repeat (Arm) domain,*1 which binds to many proteins, and have conducted many studies on that domain. One of the proteins that binds to the Arm domain is Sam68.*2 Recently, Professor Akiyama and his colleagues at the University of Tokyo have found that the binding of Sam68 to APC regulates the signaling that leads to cancerous changes. Then, his research group succeeded in determining the 3D structure of the APC-Sam68 complex by X-ray crystallography and identifying the important amino acids that form the complex. These findings will provide significant knowledge on the molecular mechanism by which mutated APC causes the development of cancers, gaining a foothold in the treatment of colon cancers. Moreover, the determination of the 3D structure of the APC–Sam68 complex will lead to the development of new anticancer drugs or new infiltration and metastasis inhibitors. This study was carried out as part of the "Targeted Proteins Research Project" and the "National BioResource Project (Drug Discovery Supporting Technology Platform)" supported by the Ministry of Education, Culture, Sports, Science and Technology. Their achievements were published online in the American scientific journal Structure on October 11 and the 3D structure of the complex also appeared on the cover. Publication: |
<<Glossary>>
*1 Armadillo repeat (Arm) domain
The armadillo repeat (Arm) domain is an approximately 40-amino-acid-long repeated sequence motif, which was first found in armadillo, a gene product in Drosophila. This motif is considered to play a significant role in the interaction among proteins.
*2 Src-associated in mitosis 68 kDa (Sam68)
Sam68 is an RNA-binding protein existing in the nucleus and is considered to transmit signals or to function in RNA metabolism. It is phosphorylated by tyrosine kinase, and its RNA binding ability is inhibited when tyrosine is phosphorylated.
*3 Wnt signal
The intracellular signaling mechanism activated by the effects of a secreted protein called Wnt on cells is the Wnt signaling pathway. The Wnt signaling pathway, in the early stages of embryonic development, is involved in important life phenomena such as morphogenesis and the determination of cell polarity. However, it is considered that the persistent activation of the Wnt signaling pathway causes the development of cancers.
*4 T-cell factor (TCF)
TCF is one of the transcription factors that binds to DNA. Its transcriptional activity is enhanced when it binds to β-catenin.
*5 Splice variant
In eukaryotes, introns in a precursor messenger RNA (pre-mRNA) are removed and exons existing before and after the introns are joined (splicing) to clip out the region where proteins are translated. In some pre-mRNAs, splicing can occur at multiple points, and, as a result, different mature mRNAs are produced from one pre-mRNA. Those various mRNAs are called splice variants, whose activation can lead to the synthesis of proteins with different levels of activity.
<<Figures>>
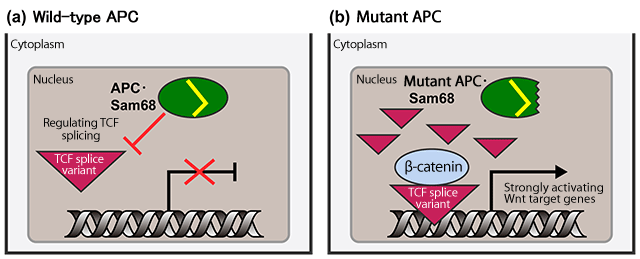
(a) Wild-type APC binds to Sam68 and the resulting complex prevents the overexpression of a T-cell factor (TCF)*4 splice variant.*5 Thus, the activation of the target genes of Wnt is prevented, resulting in the inhibition of Wnt signaling.
(b) The complex of mutant APC and Sam68 promotes the overexpression of the TCF splice variant, strongly activating the target genes of Wnt, thus, causing uncontrolled cell proliferation.
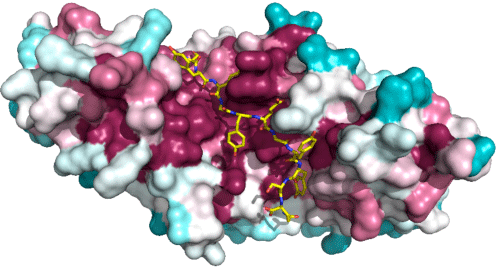
The surface of the APC molecule is colored according to the conservation of primary sequences. When the same types of amino acid residues exist in the same primary sequence of the corresponding proteins in various species of organism, that primary sequence is conserved. When the primary sequence is conserved in many species, it means that the conservation of the sequence is high. In this figure, dark purple and blue indicate high conservation and low conservation, respectively. Sam68 is represented by a stick model (red, oxygen; blue, nitrogen; yellow, carbon).
|
For more information, please contact: |
- Previous Article
- Development of High-Resolution X-ray Microscopy with Wide Field of View Capable of Element Mapping (Press Release)
- Current article
- Clarifying Three-Dimensional Structure of Adenomatous Polyposis Coli-Sam68 Complex Involved in Development of Colon Cancer (Press Release)