Commonplace ceramics can absorb a large amount of hydrogen! (Press Release)
- Release Date
- 16 Apr, 2012
- BL02B2 (Powder Diffraction)
Kyoto University
Japan Synchrotron Radiation Research Institute (JASRI)
|
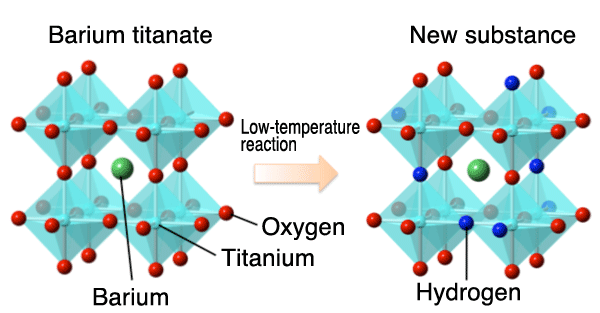
A commonplace ceramic that has supported the semiconductor industry for more than half a century has been found to possess a yet untapped potentiality: storage of a large amount of hydrogen. The study was conducted by researchers at Kyoto University’s Graduate school of Engineering (Department of Energy and Hydrocarbon Chemistry)** and Institute for Integrated Cell-Material Sciences*** in collaboration with Dr. Jungeun Kim (Associate Senior Scientist at the Japan Synchrotron Radiation Research Institute). ** Tatsunori Sakaguchi (postdoctoral student), Dr. Yoshihiro Tsujimoto (postdoctoral researcher, currently at the National Institute for Materials Science), Assistant Professor Yoji Kobayashi, and Professor Hiroshi Kageyama Barium titanate (BaTiO3) is a dielectric material that was first synthesized in the early part of the 1940s, and it has been used in every field of electronic devices. In 2007, prior to this research, Professor Kageyama et al. succeeded in realizing a local conformation of iron atoms that had long been considered unstable using a low-temperature synthesis method,*1 and the results were published in Nature. Later, in this study, the researchers applied the same low-temperature reaction scheme to barium titanate and succeeded in replacing a portion of oxygen atoms incorporated in the crystal with hydrogen atoms (figure on the right: structural determination was made using a high-intensity X-ray provided by beam line BL02B2 [for powder crystal structural analysis] at SPring-8*2). Several research attempts to introduce hydrogen atoms into barium titanate had been undertaken with limited successes: only a trace amount of hydrogen (replacing a miniscule 0.1% of oxygen) was incorporated into the substance. In contrast, the new approach reported here has enabled replacement of up to 20% of oxygen with hydrogen atoms (BaTiO2.4H0.6). The characteristics of the newly synthesized material—stability in the presence of moisture and against temperature variations—will give it an enormous potential for use as a hydrogen material (i.e. for conversion, transport, and storage of hydrogen). The academic significance of the new material lies not only in its large capacity for hydrogen storage, but also in its unique state of electric charge. The hydrogen incorporated in oxides generally takes on the form of a positively charged proton (H+). A familiar example of this is ferric hydroxide (Fe(OH)2), a major component of iron rust. In contrast to this, common sense in solid chemistry prescribes that the coexistence of a negatively charged hydride (H-) with transition metals, such as titanium, is impossible because of its powerful reducing power. The hydrogen atoms in the newly synthesized substance obtained through this study are negatively charged, disproving such traditional thought. The confirmation of the possible coexistence of H- with transitional metals may lead to the development of a new system of science, which may be designated as “oxide hydridenics,” paving the way to the future control and exploitation of hydride flow. The results of the research clearly indicated that the hydrogen atoms in the newly synthesized material have the ability to migrate inside the crystal at such low temperatures as about 400°C, indicating the possibility of hydrogen ion (H-) conductivity. The oxygen ion conductor has already attained practical use in the fuel cell electrolyte. In view of the lighter weight and better mobility of hydrogen as compared with oxygen, the utilization of hydrogen will enable, at least in principle, faster operation at lower temperatures. Considering the fact that the electrons derived from titanium atoms can simultaneously contribute to electric conduction, the material may find applications as sensors as well as fuel cells that use hydrogen as the fuel. The results of the study were published on the online publication of Nature Materials (an English scientific journal) on the 15th of April (UK time). The research was carried out as a collaborative endeavor among the following institutes: Kyoto University, Japan Synchrotron Radiation Research Institute, Kurashiki University of Science and the Arts, Tokyo Institute of Technology, National Institute for Materials Science, and the University of Rennes 1. The main part of the results reported here were obtained as a part of the project called “Exploration for Novel Superconductor and Related Functional Materials, and Development of Superconducting Wires for Industrial Applications” (principal researcher: Prof. Hideo Hosono, Tokyo Institute of Technology), which is a Funding Program for World-Leading Innovative R&D on Science and Technology of Japan Society for the Promotion of Science. The raw materials used to prepare barium titanate were obtained through the courtesy of Toda Kogyo Corp. Publication: |
<<Figures>>
Fig. (Left): Barium titanate: a semiconductive ceramic widely used in electronic materials. Fig. (Right): 20% of oxygen atoms (red) are replaced by hydrogen atoms (blue) through the process of low-temperature synthesis. Only the hydrogen atoms can migrate relatively easily through the crystal structure at such low temperatures as about 400°C. The structure was determined using the high-intensity X-ray available at SPring-8.
<<Glossary>>
*1 Low-temperature synthesis method
As easily suggested by the pottery throwing processes, oxides (ceramics) are generally synthesized at temperatures higher than 1000°C. However, the reactions can be driven at temperatures equal to, or lower than 500°C with a clever selection of reactants (e.g. calcium hydride, as used in this research). The low-temperature synthesis consists of these reactions.
*2 SPring-8
SPring-8 is a facility that generates the world's highest-performance synchrotron radiation. It is located in Harima Science Garden City in Hyogo prefecture and is owned by RIKEN. JASRI is responsible for its operation, management, and support for users. The name “SPring-8” is derived from “Super Photon ring-8 GeV.” Synchrotron radiation is the narrow and extremely powerful light that is obtained when the direction of electrons accelerated to close to the speed of light is bent using electromagnets. Research in a wide range of fields, including nanotechnology, biotechnology, and their industrial applications, has been carried out using the synchrotron radiation at SPring-8.
|
For more information, please contact: |
- Current article
- Commonplace ceramics can absorb a large amount of hydrogen! (Press Release)