Discovery of Nano-Porous Materials Capable of Memorizing/Erasing Molecule Adsorption Information (Press Release)
- Release Date
- 11 Jan, 2013
- BL13XU (Surface and Interface Structures)
Institute for Integrated Cell-Material Sciences, Kyoto University (iCeMS)
Japan Science and Technology Agency (JST)
|
A research group at Kyoto University (president: Hiroshi Matsumoto) - including Prof. Susumu Kitagawa (Director of iCeMS), Associate Prof. Shuhei Furukawa (iCeMS), and Assistant Prof. Yoko Sakata (Kobe University, affiliated with iCeMS as a postdoctoral researcher at the time of this study) - successfully synthesized a shape-memory*3 nanoporous material. The shape-memory property develops if the crystal size of a porous structure*1 is reduced to the mesoscopic regime,*2 and such material “memorizes” one structure (a guest molecule is accommodated) and is able to be “erased” through a heat treatment. The results showed, for the first time in the world, that the pore functions in porous materials can be altered by controlling the size of the material. Inorganic compounds are known to exhibit totally different functions depending on the size. For example, a bulk gold shines in a yellowish metallic color, but if ground into tiny particles down to the size of several nanometers (gold nanoparticles), the color turns to red. Such red powders have been used since olden times as a pigment for stained glass. The “size effect"*4 comes from the difference of electronic properties within the substance. On the other hand, the size effect originated from the difference of molecular flexibility has never been known up to the present. The novel size effect originates from the difference of the molecular flexibility was discovered, for the first time in the world, through the course of this research using a class of compounds called “porous coordination polymers”*5 (PCP or MOF, hereafter referred to as PCP), which are crystalline porous materials characterized by a distribution of nanoscale pores. The authors focused attention on the flexible PCP, which changes the nanopore structure in response to the accommodation of guest molecules. Its nanopore possesses a “closed” form prior to molecule adsorption, and “opens” the pore when it accommodates guest molecules. Subsequent removal of the molecules returns the nanopore to its original closed configuration. The research revealed that the flexible PCP could maintain its opened structure even after the release of the guest molecules under the condition that the crystal size is reduced down to the mesoscale, i.e. several tens of nanometers. In other words, a mesoscopic crystal can "memorize” an open structure even after the removal of the guests. It was also found that a heat treatment could turn the open structure back into the closed structure, indicating the possibility of synthesizing shape-memory nanopores, i.e. the pore structure enables the memorizing and erasing of information regarding molecular adsorption. The findings obtained through this research are expected to play a conducive role, not only for porous material research, but also for enhancing research on molecular behavior within organic crystals, such as protein crystals and organic polymer crystals, as well as for the development of separation technology. In this research, the molecular adsorption process was traced by the X-ray diffraction method using the high-intensity beam line BL13XU available at SPring-8, leading to elucidation of the relationship between molecular adsorption and structural changes in the PCP. The research was conducted as a part of the “Kitagawa Integrated Press Project” *. The research results were reported in Science (a U.S. science journal) on the 11th of January, 2013. Publication: |
<<Figures>>
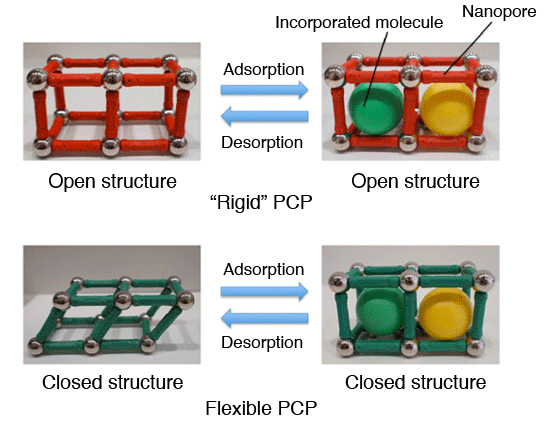
Schematic representations of “Rigid” PCP and flexible PCP The rigid PCP has a fixed configuration capable of incorporating molecules in its interstitial pores, while the flexible counterpart incorporates molecules as it changes conformation, from a closed to open structure.
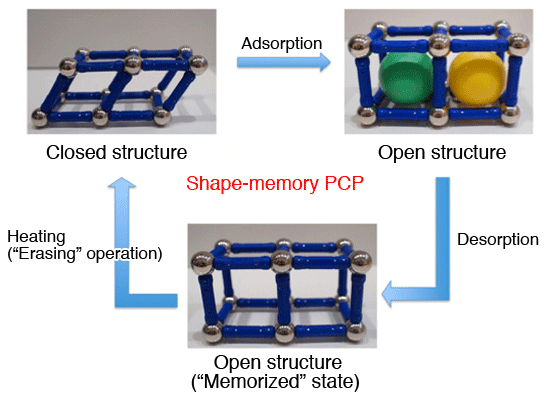
Schematic representation of shape-memory PCP. Shape-memory PCP, like flexible PCP, changes its conformation - from closed to open - by incorporating molecules in its fine pores. It maintains the open structure even after the molecules have been removed, or it has memorized the crystal configuration. Subsequent heating can restore the original closed configuration (“delete” operation).
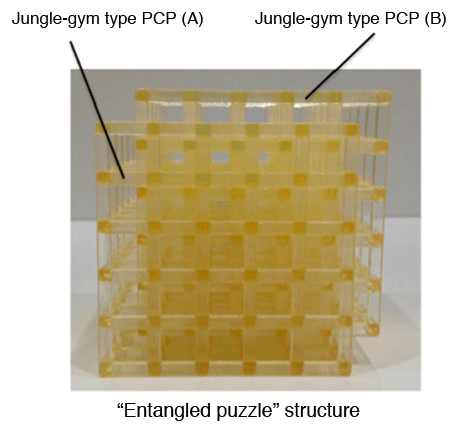
A model of an “entangled puzzle” structure. It is characterized by an interpenetrating grid structure of jungle-gym B into A.
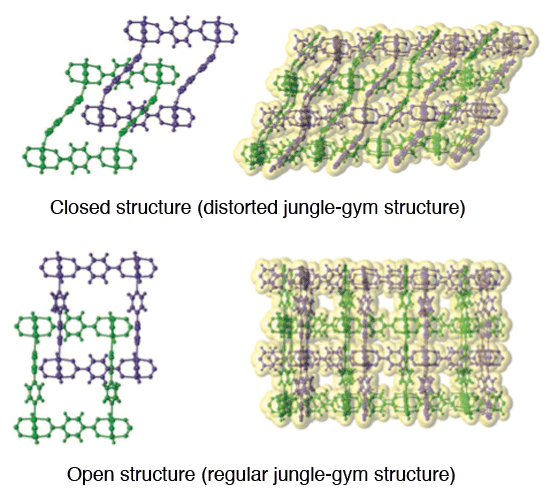
Molecular structure of an entangled-puzzle lattice configuration as revealed by single crystal X-ray diffraction analysis. It is characterized by an interpenetrating grid structure of jungle gym B (purple) into jungle gym A (green). It takes an open structure when the molecules are in interstitial positions (adsorbed molecules are not shown, for clarity), and restores its closed configuration when the molecules are removed.
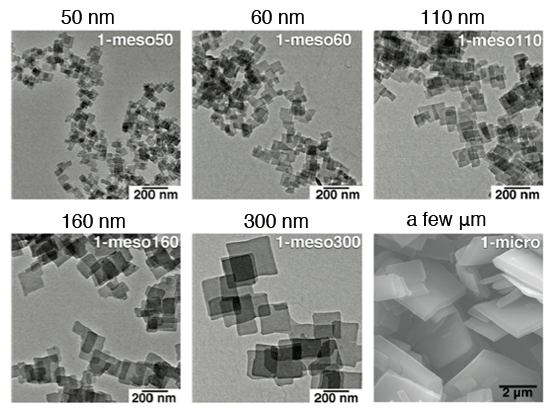
Electron microscope images of the synthesized crystals. The crystal sizes are controlled in the ranges from 50 nanometers to several micrometers.
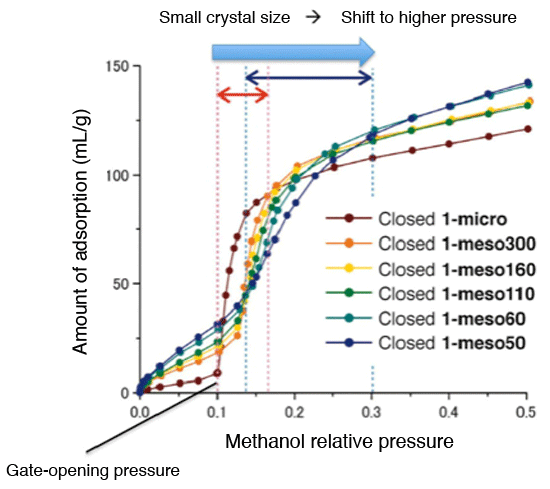
Methanol adsorption by size-controlled crystals. As the crystal size becomes smaller, the gate-opening pressure gradually shifts to the higher pressure region, indicating that the smaller the crystal size, the more rigid it will be.
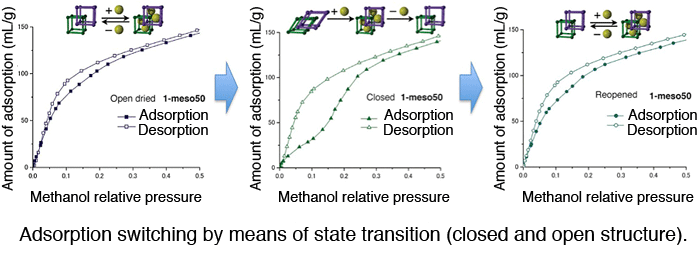
Adsorption switching by means of state transition (closed and open structure). Methanol adsorption switching using 50-nanometer crystals. Left: When an open-structure crystal is brought into contact with methanol, instantaneous uptake of methanol takes place in the low pressure region. Middle: Adsorption measurement was then carried out after a state transition (open to closed) effected by a heat treatment. The figure shows structural change from an open to closed state (gate-opening pressure is shown). Because of the shape-memory property, the open structure is maintained even after desorption. Right The crystal is brought into contact with methanol again. The adsorption profile is the same as the one shown on the left, indicating the maintenance of the open structure.
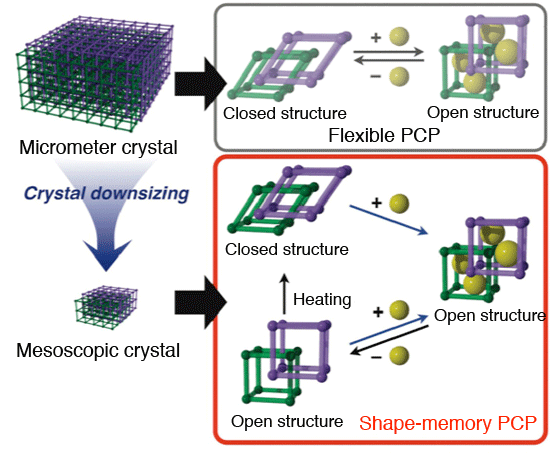
Transitions summary. Reducing the crystal size promotes property transition: from flexible PCP to shape-memory PCP. This transition is effected through the rigidifying process of the crystal structure as it becomes smaller, enabling it to maintain the open structure even after desorption of the molecules.
<<Glossary>>
*1 Porous structure
A material with a multitude of fine pores. This class of materials is widely used for such applications as adsorption, separation, and catalyst.
*2 Mesoscopic regime
The term pertains to a size regime, between nanoscopic and macroscopic (typically from several tens to several hundreds of nanometers). Molecules have sizes of up to several nanometers, and many cellular functions express themselves in macroscopic domains. The mesoscopic regime is considered important for research in a variety of fields, including the understanding of life phenomena and the cooperative behavior of molecules in a crystal structure. Meso-science, as well as stem-cell research (ES/iPS cells, etc.), represent the key concept for iCeMS activities. Since 2011, the U.S. Department of Energy (DOE) has implemented measures to probe the potential of meso-science,A including discussion in the DOE advisory committeeB followed by the publication of its report,C and presentations in international conferences.D These moves have led to the launch of dedicated portal site, and have been drawing attention to this area of science.
*3 Shape-memory
The shape-memory effect refers to a phenomena in which a material, after being deformed through a certain operation (pressure, temperature, etc.), maintains a deformed shape until it recovers its original shape through the application of a different operation (temperature, light, etc).
*4 Size effect
A phenomena in which the functions of a material change depending on its size. Gold and semiconductors are known to exhibit size effect.
*5 Porous coordination polymers (PCP or MOF)
Crystalline metal complex whose lattice structure incorporates metal ions and organic ligands (a jungle-gym structure). This class of compounds intrinsically includes a distribution of nanopores, and is expected to have a wide variety of applications for gas storage, gas separation, catalysts, and sensors.
|
For more information, please contact: Prof. Susumu Kitagawa (Kyoto University) |
- Current article
- Discovery of Nano-Porous Materials Capable of Memorizing/Erasing Molecule Adsorption Information (Press Release)