Magnesium binding and sarcolipin regulation of calcium pump as revealed by X-ray crystallography (Press Release)
- Release Date
- 04 Mar, 2013
- BL41XU (Structural Biology I)
The University of Tokyo
Points of presentation
• Key research findings: The structure of the calcium pump ready to bind calcium ions was determined by X-ray crystallography.
• Novelty of research: By identifying the conditions that allow binding of magnesium ions and a regulatory protein sarcolipin, the scientists have overcome one of the biggest challenges in “Elucidation of the mechanism of calcium pumping based on the atomic structure.”
• Impact on the society and perspective: Calcium pumps and sarcolipin play important roles in muscle-based thermogenesis, and a lack of sarcolipin is reported to cause hypothermia and an increase in body fat. The methods developed in this study will contribute to large-scale production and crystallization of large mammalian membrane proteins, which are therapeutic targets but have been difficult to produce.
|
The research group led by Chikashi Toyoshima (professor) of the Institute of Molecular and Cellular Biosciences, the University of Tokyo, determined the structure of the calcium pump protein*1 in a state ready to bind calcium ions by X-ray crystallography at SPring-8. This state was one of the biggest challenges in “Elucidating the mechanism of the calcium pump based on the atomic structure,”*2 which has been the research objective of Professor Toyoshima. He and his colleagues observed that magnesium ions, abundant in cells, prepare the pump protein for binding calcium ions. Also, sarcolipin,*3 a regulatory protein of calcium pumps, was identified in the crystal structure, stabilizing the state of the calcium pump ready to accept new calcium ions. Sarcolipin, reported to play an important role in muscle-based thermogenesis and fat burning, is implicated in the regulation of cardiac contractility together with a closely related protein phospholamban.*4 Sarcolipin, therefore, is a therapeutic target. The research group also developed a new method for large-scale production and purification of large mammalian membrane proteins*5 and succeeded in crystalizing them This method will significantly promote researches on large mammalian membrane proteins, which are important for drug development. Publication: |
<<Figures>>
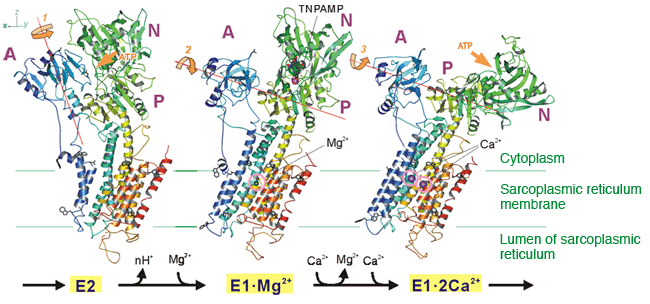
In E2, after the release of calcium ions to the lumen of sarcoplasmic reticulum, calcium pump shows low binding affinity for calcium ions due to bound protons. High affinity for calcium ions is gained by binding a magnesium ion, which is 1000 times more abundant than calcium ions (E1·Mg2+). Then, two calcium ions bind to the binding site and displace the magnesium ion (E1·2Ca2+). This binding signal is transmitted to the ATP binding site to cause phosphorylation.
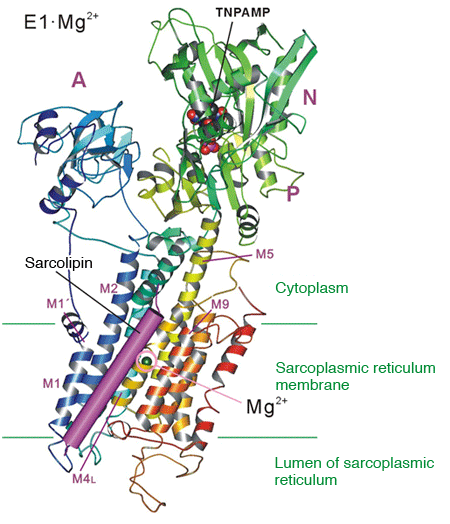
Sarcolipin, a regulatory protein of calcium pumps, binds to the cleft between the transmembrane helices M2 and M9 and stabilizes the E1·Mg2+ state.
<<Glossary>>
*1 Calcium pump proteins
Large membrane proteins that transport calcium ions against a concentration gradient across biological membranes using the energy of adenosine triphosphate (ATP).*6 Calcium ions are transported by changing the calcium-binding sites from high affinity (E1) to low affinity (E2) for calcium, at the same time from facing the inside (E1) to outside (E2) the cell. Because the calcium pump occupies more than half of the membrane proteins of the sarcoplasmic reticulum*8 in skeletal muscle and consumes a large amount of ATP, it has been thought as a heat source for maintaining body temperature. In fact, the heat-generating organs of fish that dive deep in the ocean, such as swordfish, use calcium pumps for thermogenesis.
*2 Elucidating the mechanism of calcium pump based on the atomic structure
Professor Toyoshima was awarded the Asahi Prize 2009 for this research achievement.
*3 Sarcolipin
A membrane protein consisting of 31 amino acid residues, predominantly present in sarcoplasmic reticulum in skeletal and atrial cardiac muscles. It regulates the activity of calcium pumps and may play an important role in muscle-based thermogenesis for maintaining body temperature. According to a study published in Nature Medicine in autumn 2012, sarcolipin is implicated in fat burning and its removal causes a gain of body fat in mice. It is also reported that when mice are fed a high-fat diet, sarcolipin expression in skeletal muscles increases to enhance the metabolism. Sarcolipin may become a therapeutic target for obesity in the future.
*4 Phospholamban
A membrane protein consisting of 52 amino acid residues, mainly present in the sarcoplasmic reticulum in cardiac ventricles. It works as an inhibitor of the calcium pump, but the inhibition is relieved when excitation occurs. Thus, phospholamban “reserves” contractility for excitation. Phospholamban has been attracting attention as a target molecule for treating cardiac diseases.
*5 Membrane proteins
Collective term for proteins present in biological membranes. Membrane proteins attached to the surface of biological membranes are called peripheral membrane proteins and those embedded in the membranes are called integral membrane proteins. Ion pumps*7 are integral membrane proteins. Membrane proteins are known for their difficulty in large-scale production and crystallization. In particular, large mammalian membrane proteins cannot be produced in Escherichia coli, and even verifying their functions is difficult. While more than half of the future therapeutic targets are membrane proteins, the difficulty in their large-scale production has been a great obstacle. In this research, the scientists succeeded in the large-scale production of large mammalian membrane proteins with a method using cells derived from African green monkey kidneys (COS cells).
*6 Adenosine triphosphate (ATP)
A substance known as the energy currency in living organisms. The chemical energy released when ATP breaks down to adenosine diphosphate (ADP) and phosphate is the energy source for most biological reactions, including muscle contraction.
*7 Ion pumps
There are differences in the concentration of ions such as sodium, potassium, and calcium inside and outside a cell. Living organisms utilize the concentration differences for signaling or as an energy source for driving transport proteins. Ion pumps are proteins integrated in biological membranes, maintaining these concentration differences. The chemical energy from ATP (light energy for some pumps) is used as an energy source required for transporting ions against the concentration gradient.
*8 Sarcoplasmic reticulum
A sac-like structure surrounding a myofibril in muscle cells. The sarcoplasmic reticulum is a reservoir of calcium ions. Muscle contraction starts when the calcium ions stored in the sarcoplasmic reticulum are released, and the muscle is relaxed when the calcium ions are pumped back into the sarcoplasmic reticulum by calcium pumps.
*9 Center for Structural Biology of Challenging Proteins, Institute of Molecular and Cellular Biosciences, The University of Tokyo
A research institute established in 2010. Although most of the important therapeutic targets are membrane proteins, the number of registered three-dimensional structures of membrane proteins accounts for only 0.36% of the total protein structures in the Protein Data Bank. Therefore, this research institute is aimed at intensively investigating the membrane proteins that may become therapeutic targets and lead to breakthroughs in drug development.
|
For more information, please contact: |
- Previous Article
- World’s First Discovery of Anomalous Pressure-Dependent Conductivity of Organic Semiconductors (Press Release)
- Current article
- Magnesium binding and sarcolipin regulation of calcium pump as revealed by X-ray crystallography (Press Release)