In Silico Drug Discovery Based on the Identification of a Novel “Druggable” Surface Pockets on the ras Oncogene Products (Press Release)
- Release Date
- 30 Apr, 2013
- BL38B1 (Structural Biology III)
- BL41XU (Structural Biology I)
Kobe University
|
A research group led by Tohru Kataoka (professor) and Fumi Shima (associate professor) of Kobe University Graduate School of Medicine discovered substances (low molecular weight compounds) that exhibit anti-tumor activity by inhibiting the functions of the ras oncogene products (Ras),*1 which are the cause of approximately 20% of human cancers. These compounds may serve as a scaffold for the development of molecularly targeted anti-cancer drugs (molecularly targeted drugs)*2 for Ras. Their achievement was published online in Proceedings of the National Academy of Sciences of the United States of America (PNAS). Despite its importance as an anti-cancer drug target, there has been no effective molecular targeted therapy for Ras at present (Fig. 1). Since the first determination of the crystal structures of Ras in the late 1980s, researchers have considered that no effective strategy would be available for the development of Ras inhibitors because of the absence of well-defined surface pockets suitable for drug binding (Fig. 2). According to a patent search, the Kobe family compounds have not been registered as anti-cancer drugs. Kobe University applied for a national patent for these compounds (including the screening method for Ras inhibitors) as the candidates for the development of new anti-cancer drugs in 2011 and will soon apply for a US patent. Major global pharmaceutical companies such as Genentech*6, an affiliate of Roche,*5 and Abbott Laboratories*7 have devoted much effort to exploring and developing Ras inhibitors. Their achievements have recently been published in leading scientific journals such as PNAS, intensifying the competition in this research field. However, the novel inhibitors discovered in this study are quite different from theirs in terms of the mechanism of action. Moreover, the novel inhibitors show stronger biochemical and cellular activities and, furthermore, they exhibit a significant anti-tumor effect in the mouse xenograft model of human colon cancer unlike the compounds reported by the companies (Fig. 5). Although the inhibitory activity is not particularly potent for the clinical application at present, the Kobe family compounds may serve as a lead scaffold for the development of the world’s first anti-cancer drug targeting Ras. Prior to the publication of this research achievements, a joint press conference attended by representatives of seven media outlets was held at Kobe University Graduate School of Medicine on 25 April 2013, as shown in the picture. Picture: Press conference held at Kobe University Graduate School of Medicine
(Kusunoki Campus) attended by seven media outlets. Starting from the left, Dr. Kumasaka, Associate Professor Shima, and Professor Kataoka. Publication: |
<<Figures>>
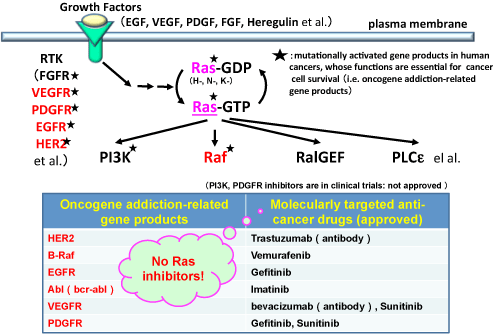
Although a number of molecularly targeted anti-cancer drugs against intracellular signaling molecules related to oncogene addiction are currently available on the market, there is no effective drugs targeting Ras.
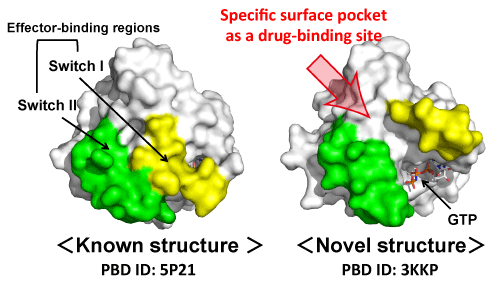
already-known structures of Ras used in this research
The newly determined structure (right, M-Ras) possesses a surface pocket between the two switch regions which are important for recognition of various effectors, including Raf, PI3K and PLCε (Fig. 1). In contrast, the already-known structure (left, H-Ras) does not possess a pocket in the corresponding region.
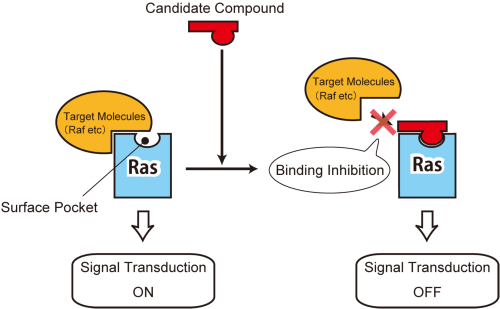
Ras-mediated cancer signaling
The compounds which bind to the surface pocket of Ras inhibit the interaction between Ras and its effectors, thereby preventing signals essential for cancer cell growth and proliferation.
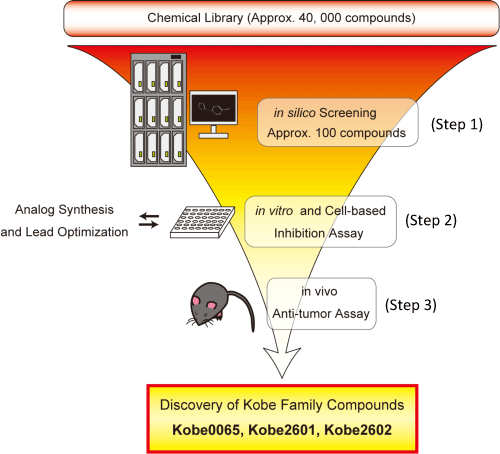
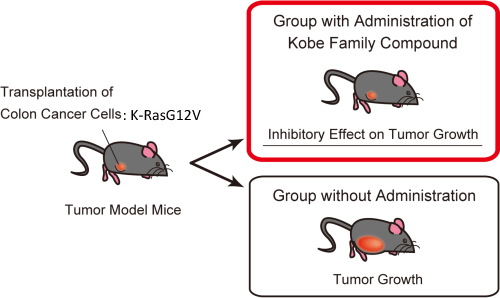
(Step 1) Screening for the candidate compounds which bind to the surface pocket of Ras by computer-docking simulation, (Step 2) examination for inhibitory activity of the selected compounds in vitro and in cellular system using cancer cells carrying active Ras mutations (H-, K-RasG12V), and (Step 3) examination for in vivo anti-tumor activity of the compounds using a xenograft model in the nude mice transplanted with human colon cancer cells bearing K-RasG12V.
When the Kobe Family compounds were administered in the nude mice transplanted with colon cancer cells bearing activated Ras (K-RasG12V), a significant inhibitory effect of the compounds on tumor growth was observed compared to the control.
<<Glossary>>
*1 ras oncogene products (Ras)
The name “ras” is an abbreviation of “rat sarcoma virus” from which it was discovered. There are three ras oncogenes, H-ras, K-ras and N-ras in mammals. Their products, collectively called Ras, belong to a family of small G proteins, i.e. guanosine triphosphate (GTP)-binding proteins, and regulate intracellular signaling pathways controlling cell growth, differentiation, apoptosis and cell mobility. They exist in the genome of normal cells as the ras proto-oncogene and are converted to the ras oncogenes when the activities of their products are hyperactivated by point mutations.
*2 Molecular targeted anti-cancer drugs (molecularly targeted drugs)
Drugs designed to detect a specific property of cancer cells at the level of signaling molecules and to target such molecules are collectively called molecularly targeted drugs. Different from chemotherapeutic drugs, which are designed to kill normal cells as well as cancer cells, molecularly targeted drugs are expected to achieve better therapeutic effects with less side effects because they affect cancer cells only.
*3 Structure-based drug design
Rational drug design is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. Structure-based drug design, aided by computer modeling techniques, involves the design of small organic molecules that are complementary in shape and charge to the biomolecular target with which they interact and therefore will bind to it.
*4 xenograft
A graft of tissue transplanted between animals of different species; it may be concordant, occurring between closely related species, in which the recipient lacks natural antibodies specific for the transplanted tissue, or discordant, occurring between members of distantly related species, in which the recipient has natural antibodies specific for the transplanted tissue. The nude mouse, with an inhibited immune system due to a greatly reduced number of T cells, is valuable to research because it can receive many different types of tissue and tumor grafts, as it mounts no rejection response. These xenografts are commonly used in research to test new methods of imaging and treating tumors.
*5 Roche
A global pharmaceutical and health-care company with its head office located in Basel, Switzerland. Established in 1896, Roche built its foundation by manufacturing vitamins. Since the 1950s, it has developed and sold diazepam (sold under the trade name of Cercine in Japan), which has become the global standard for anxiolytic drugs. It has dominated the market of anxiolytic drugs and generated a significant profit for Roche. Since the 1990s, Roche has focused on the development of anticancer drugs.
*6 Genentech
A major and world-leading biopharmaceutical manufacturer in the United States. When Roche first bought Genentech in 1990, it was a small biotechnology company in San Francisco, the United States, with some promising anti-cancer drugs under study but lacking the funds to continue development. However, because of the enormous financial power and the expertise of Roche in clinical development, Genentech now has many anticancer drugs that have become huge successes such as Avastin for breast, lung, and colorectal cancers, and Herceptin for breast cancer.
*7 Abbott Laboratories
A pharmaceutical and health-care company in Illinois, the United States, which was established in 1888. It develops and provides a wide range of medicinal products such as HIV test agents, Humira for rheumatoid arthritis, Kaletra for HIV, and the antiepileptic drug Depakote.
|
For more information, please contact: Associate Prof. Fumi Shima (Kobe University Graduate School of Medicine) |
- Previous Article
- Ultra-high pressure polymorph of silica discovered in lunar meteorite: Record of asteroid collision on the moon 2.7 billion years ago (Press Release)
- Current article
- In Silico Drug Discovery Based on the Identification of a Novel “Druggable” Surface Pockets on the ras Oncogene Products (Press Release)