Wing-beat mechanism revealed by ultrafast X-ray movies (Press Release)
- Release Date
- 26 Aug, 2013
- BL40XU (High Flux)
Japan Synchrotron Radiation Research Institute (JASRI)
|
Japan Synchrotron Radiation Research Institute (JASRI) has succeeded in deciphering the mechanism of high-frequency wing-beat of insect, by irradiating the chests of beating insects and recording ultrafast X-ray movies at a rate of 5,000 frames/s. Smaller insects beat their wings at higher frequencies, and in the case of mosquitoes, they beat their wings 500 times a second. They can beat at such high frequencies because their flight muscles undergo self-sustained oscillations while they remain constantly activated. These self-sustained oscillations are caused by the process of stretch activation (SA, a process in which a muscle generates a large force when externally stretched). The molecular mechanism of SA has been unknown in spite of researches for decades. It has been shown recently that insects express many flight muscle-specific proteins, and a prevailing hypothesis is that these proteins are responsible for SA. In this study, the researchers have succeeded in recording diffraction patterns from beating bumblebees at an unprecedented rate of 5,000 frames/s, by irradiating their thoraces with intense X-rays from the high-flux BL40XU beamline of SPring-8*1. The bumblebees beat their wings 120 times a second (8 ms per wing-beat cycle), so that 40 frames were taken for each wing-beat cycle. This allowed detailed analyses of the movement of proteins in the flight muscles. In addition, it was possible to estimate the changes of length and force of muscles from the analyses of diffraction patterns. An important outcome of the study is the detection of a signal that precedes each SA event, in the right timing for triggering SA. This signal is inferred to come from a deformation of myosin, the contractile protein responsible for force generation. The deformation-induced generation of large force by myosin has long been reported for vertebrate skeletal muscle. Thus, insect flight muscle relies on a general property of muscle also found in vertebrates, rather than their specific proteins, in creating SA. Because of the common mechanism shared by insect flight muscle and vertebrate muscles, insect flight muscle is expected to serve as a model material to better understand the functions of skeletal and cardiac muscles of vertebrates. This is particularly important for studying cardiac muscle, because of the suggested role of SA in heartbeat. This work was supported by Grant-in-Aid, Ministry of Education, Culture, Sports, Science and Technology, No. 23612009. Publication: |
<<Figures>>
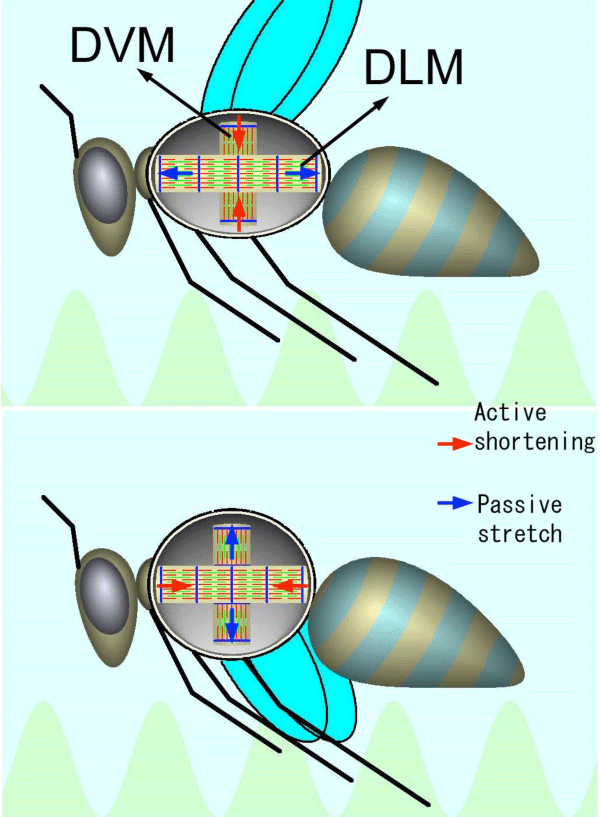
There are two antagonistic flight muscles in the thorax, dorsal longitudinal muscle (DLM) and dorsoventral muscle (DVM). They are arranged in a way that when one contracts, the other is stretched. The two muscles alternately stretch each other, causing SA, enabling continuous wing-beats.
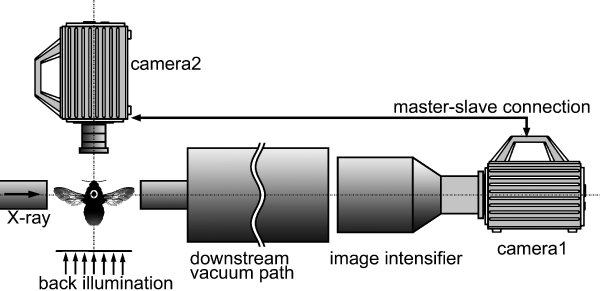
Two fast CMOS video cameras*2 are connected in a master-slave fashion*3, and they synchronously record diffraction patterns and wing-beats.
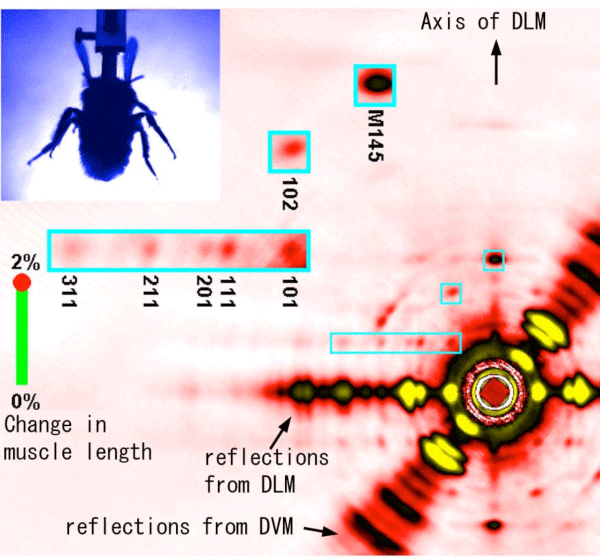
The yellow dots mark the software-recognized positions of wings.
Data from all the bees used in the experiment have been summed after rotating the patterns so that the axis of DLM fibers is vertical. The regions of interest are magnified in blue boxes (all of the reflections in the boxes come from DLM).
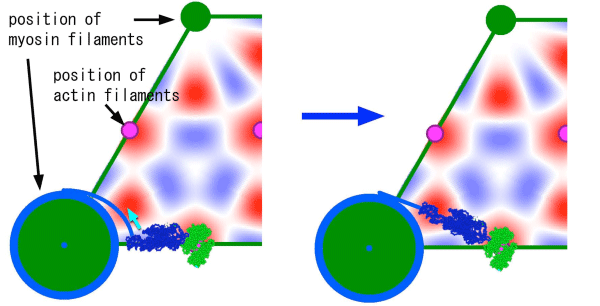
This is a magnified view of the cross section of muscle. The blue irregular-shaped object is the myosin molecule, and the green one is the actin molecule. The intensity change of the 111 spot that precedes an SA event is explained if the myosin molecule undergoes a twisted motion as shown (from left to right).
Figs. 2-5 have been modified from the article in Science.
<<Glossary>>
*1 SPring-8
A large synchrotron radiation facility that generates the highest-quality synchrotron radiation, located in Hyogo prefecture, Japan. Owned by Riken, and operated by JASRI. The nickname SPring-8 is short for Super Photon ring-8 GeV.
Synchrotron radiation refers to the strong and highly oriented electron magnetic waves generated when the orbit of electrons, accelerated to a near-light speed, is bent by magnetic field. Applications of the synchrotron radiation produced by SPring-8 includes nanotechnology, biotechnology and industrial use.
*2 fast CMOS video camera
A video camera using a CMOS (Complementary Metal-Oxide Semiconductor) area sensor chip. Compared with a conventional CCD (Charge-Coupled Device), faster recording is possible because each pixel has its own amplifier.
*3 Master-slave connection
A method to operate two instruments connected in tandem. One of the instruments (slave) is operated by the clock generated by the other (master), enabling complete synchronization of the two instruments.
|
For more information, please contact: |
- Previous Article
- New X-Ray Measurement Method for Minimizing Deterioration of Protein Crystals (Press Release)
- Current article
- Wing-beat mechanism revealed by ultrafast X-ray movies (Press Release)