Clarification of Recognition Mechanism of Pathogenic Invasion by Toll-like receptor 9 Inducing Innate Immune Responses -Leading to development of antiviral drugs and vaccines- (Press Release)
- Release Date
- 10 Feb, 2015
- BL41XU (Structural Biology I)
University of Tokyo
Osaka University
Japan Science and Technology Agency
Key Points
• The crystal structure of the protein Toll-like receptor 9 (TLR9) that recognizes the invasion of pathogen into the body and induces innate immune responses was clarified.
• The binding modes of TLR9 to a pathogenic DNA that activates TLR9 and an inhibitory DNA that inactivates TLR9 were elucidated.
• The clarification of the mechanism of pathogenic DNA sequence recognition by TLR9, which is an important target in drug discovery, will lead to the development of antiviral drugs, antiallergy drugs, and vaccines that regulate TLR9.
|
The crystal structure of TLR9, which recognizes pathogenic invasion and induces innate immune responses, was clarified in detail for the first time in the world. This was achieved by a research group led by Toshiyuki Shimizu (professor) and Umeharu Ohto (instructor) of the Graduate School of Pharmaceutical Sciences, the University of Tokyo; Kensuke Miyake (professor) and Takuma Shibata (assistant professor) of the Institute of Medical Science, the University of Tokyo; and Susumu Uchiyama (associate professor) and Elena Krayukhina (specially appointed researcher) of the Graduate School of Engineering, Osaka University. Publication: |
<<Figures>>
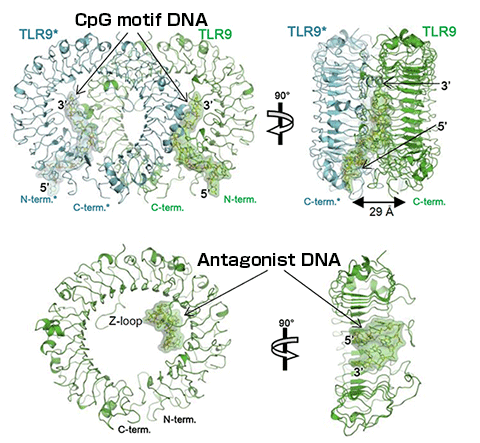
(Top) Binding mode between TLR9 and DNA sequence containing CpG motif.
(Bottom) Binding mode between TLR9 and antagonist DNA sequence.
One of the TLR9 molecules forming a dimer is indicated in green and the other in blue. The CpG motif has an elongated structure and binds to the TLR9 dimer at two sites (2:2 complex). The antagonist DNA binds to the inner surface of the TLR9 horseshoe-like structure in the form of a loop (1:1 complex).
<<Glossary>>
*1 Interferon
A protein secreted by cells of the immune system in response to the invasion of pathogens such as bacteria and viruses. Interferon suppresses viral growth and activates the immune system.
*2 CpG motif
A DNA sequence containing phosphodiester-linked cytosine and guanine. The CpG motif is often methylated in mammals, but it is unmethylated in bacteria and viruses. The unmethylated CpG motif strongly activates TLR9 and induces various immune responses.
|
For more information, please contact: |
- Previous Article
- Clarification of Crystal Structure of Protein That “Removes” Causative Substance of Alzheimer’s Disease (Press Release)
- Current article
- Clarification of Recognition Mechanism of Pathogenic Invasion by Toll-like receptor 9 Inducing Innate Immune Responses -Leading to development of antiviral drugs and vaccines- (Press Release)