Scientists Solve Structures of Grouper Virus Effective Vaccine Could Be Developed Using This Structure
- Release Date
- 12 Nov, 2015
- BL12B2 (NSRRC BM)
- BL44XU (Macromolecular Assemblies)
|
A team of scientists from National Synchrotron Radiation Research Center (NSRRC) and National Cheng Kung University (NCKU) has discovered a unique structure from deadly grouper viruses for the first time in the history. Chun-Jung Chen (NSRRC), Nai-Chi Chen (NSRRC) and Tzong-Yueh Chen (NCKU) used Taiwan Light Source in Taiwan and SPring-8 facilities in Japan to determine the three-dimensional crystal structure of Grouper Nervous Necrosis Virus (GNNV) and discovered the protrusion domains on its surface. This unprecedented research result was published in the prestigious journal PLoS Pathogens. Publication |
Synchrotron light makes the invisible visible
Taiwan, also known as the kingdom of grouper fish farming, is a major exporter of the fish, yielding 2,500 tones of groupers and earning over NT$8 billion in production value a year. Several grouper virus outbreaks, however, have caused serious damage and loss to fish farming in recent years.
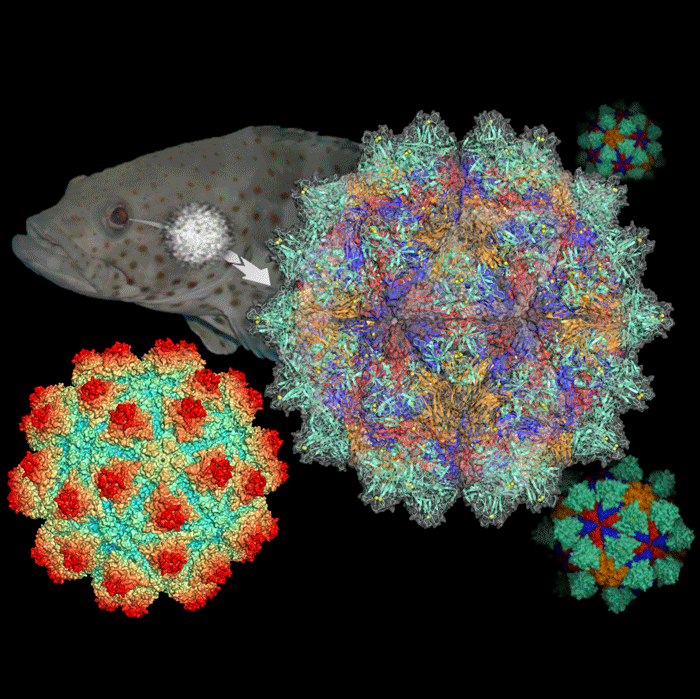
The research team discovered that 60 large protrusion domains symmetrically cover on the entire surface of the grouper virus. Each protrusion domain is composed of three capsid proteins. This protrusion domain is the key to understand the complicated structure of the virus. With this discovery, we might be able to understand the mechanism how the viruses can utilize this “key” to interact with the receptors on the cell membrane of fishes for viral infection.
“The synchrotron light is 1,000 times brighter than the conventional X-ray. Its spatial resolution is 10 times higher than an electron microscope. Scientists are able to analyze this virus structure down to 0.36 nm atomic resolution, about 0.00003% of the diameter of a hair,” Chun-Jung Chen pointed out, “Synchrotron light makes these invisible ‘keys’ visible.”
New vaccine could revolutionize fish farming
Groupers produce a high economic value but suffer frequently from virus outbreaks and hazardous environments. Hence, only less than 1% larva fish can survive and grow to adult fish. Current vaccines on the market are expensive but less effective because of the low specificity.
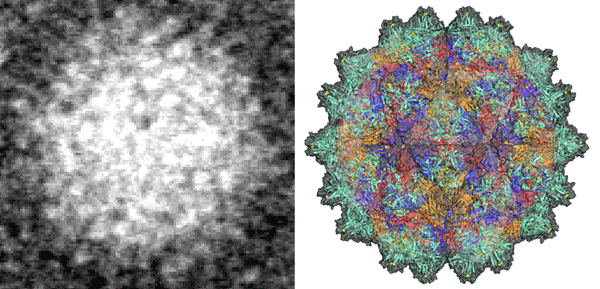
Tzong-Yueh Chen commented, “In the past, only the obscure image of this virus was available by the electron microscope, which could not provide sufficient information to develop the vaccines with the high specificity. Using the detailed “key” structure as the antigenic epitope, researchers can develop a more effective vaccine to prevent the groupers from virus infections.”
“The most essential and difficult part in this long-term research was growing high-quality virus crystals by the method of protein crystallography. It was just like raising kids, which needs great patience and care. It took us five years to grow and screen hundreds of potential crystals, and finally we obtained the diffraction data at high resolution,” Nai-Chi Chen expressed.
Taiwan Photon Source for life science
Chun-Jung Chen added, “Protein crystallography combined with the synchrotron X-ray light is very critical in determining this huge and complicated virus structure. The diameter of GNNV particle is about 30 nm, which is 10 times bigger than general proteins. It is not only challenging to grow such big and quality crystals, but it also requires high intense X-rays to acquire the data at high resolution.”
NSRRC is now constructing beamlines at the Taiwan Photon Source (TPS), which will be open to users in the coming year 2016. The synchrotron light of the TPS is 10,000 times brighter than that of the current TLS, making it possible to resolve images with much higher resolution. Even with crystals of the small size or insufficient quality, the ultra-bright X-ray of the TPS can overcome the experimental limitation and efficiently resolve the structures, and thus shorten the research period dramatically. Moreover, the synchrotron light of the TPS can be utilized in studying more complicated protein structures and developing targeted drugs.
Fig. 1: Grouper Nervous Necrosis Virus infects the nerve center of fish through eyes and brains.
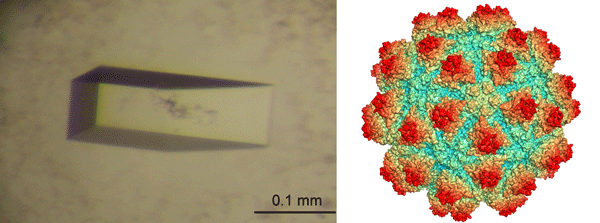
Fig. 2: (Left) The crystal of Grouper Nervous Necrosis Virus; (right) the protrusion domains (red) symmetrically distributed on the entire surface of Grouper Nervous Necrosis Virus
Fig. 3: (Left) The obscure image from electron microscope; (right) the fine atomic-resolution image of the virus structure from synchrotron light
Fig. 4: (Left) Protein crystallography end station at the Taiwan Light Source; (right) protein crystallography laboratory
|
《Contact Information》 |
- Previous Article
- Photoelectron diffraction measurements of gaseous molecules aligned in one direction: towards ultrafast molecular imaging(Press Release)
- Current article
- Grain boundary sliding as the major flow mechanism of Earth’s mantle (Press Release)