New Protein Discovery Solves Myth of Bacterial Drug Resistance (Press Release)
- Release Date
- 30 Dec, 2015
- BL12B2 (NSRRC BM)
- BL44XU (Macromolecular Assemblies)
New findings on bacteria of Typhoid fever
An international team of Taiwan and Malaysia has discovered unprecedentedly a unique structure of an outer-membrane protein from bacteria that causes the contagious Typhoid fever. Chun-Jung Chen (NSRRC), Hong-Hsiang Guan (NSRRC) and their collaborators used the experimental stations at the Taiwan Light Source (TLS) in Taiwan and SPring-8 in Japan as a crucial process to determine this important structure. It took four years to figure out the 3D structure of ST50, the outer-membrane protein, from Salmonella Typhi. The research team discovered the key of how ST50 can excrete the antibiotics outside the pathogen and result in antibiotic resistance. The research results have been published in Scientific Reports.
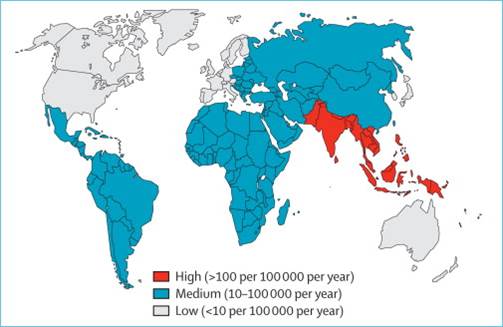
Typhoid fever is a highly contagious bowel disease, which causes persistent high fever, dry cough, headache, stomach flu, intestinal perforation, hemorrhage, rashes, and even death. Over 21 million cases are reported globally and the disease resulted in about 200 thousand deaths each year. In Asia, it is prevalent particularly in Indonesia, China, Thailand, Vietnam, and the Philippines. As there are about 9 million Taiwanese travelers visiting China and Southeast Asia a year, some could be infected with the disease during their travels.
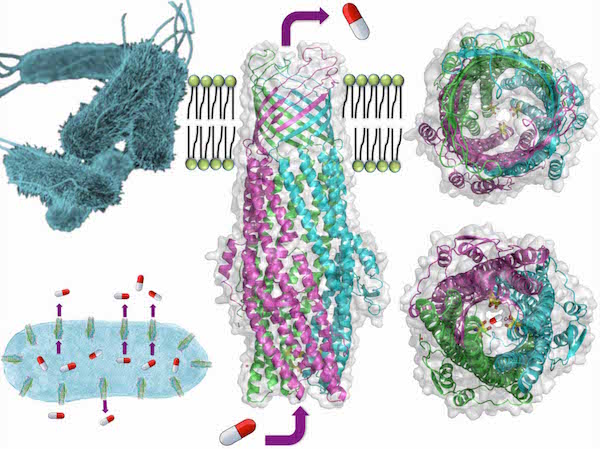
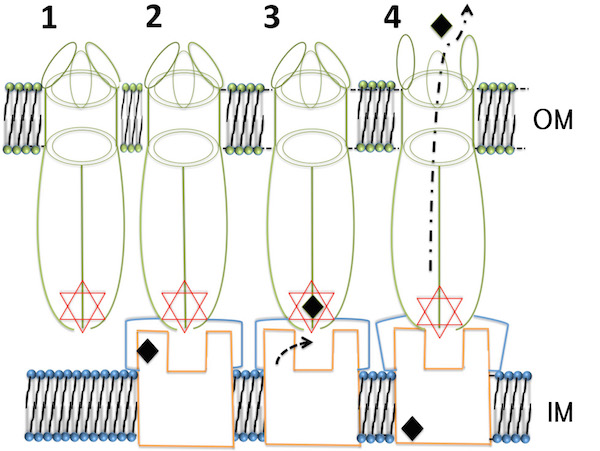
This team discovered that the ST50 proteins, located on the surface of bacteria, equip a fascinating structure to function as an efflux pump, which excretes the antibiotics out of bacteria. A new discovered “D-cage”, which comprises six aspartic acids at the entrance of the pump, captures antibiotics and other poisonous metabolites or xenobiotics before these toxic elements can be transported out through the protein channel. Such a transport mechanism makes the bacteria drug-resistant.
Antibiotics are commonly used to kill bacteria, but currently it is less effective as expected due to drug resistance. Chen pointed out, “Now that we have revealed the structure of this efflux pump at resolution 2.98 Å, 300 thousand times smaller than the diameter of single hair, it could pave the way for the future development on anti- drug-resistant treatment.”
Chen continued, “Traditional drug-development process from the scratch is a billion-dollar gamble. Scientists start with screening 5 to 10 thousand drug compounds. 250 drug candidates are subsequently selected for animal tests, and finally only less than 10 compounds enter the stage of human clinical trials. The process of a new drug discovery usually takes 0.8 to 2 billion USD and 1.5 decade, which is costly and inefficient. However, with the detailed structures of key proteins in pathogens analyzed by the synchrotron light, we can design the better structure-based target-drugs, which shortens the time and lowers the cost dramatically.”
Proteins are the origin of life, and synchrotron sheds light on them
Proteins are the key components of life organisms and act as the executing machineries in cells. Each protein essentially folds into its own special structure and shape to reflect its unique function. Proteins could be the receptors on the cell membrane and the messengers among or inside the cells, responsible for signal transduction, energy transfer and function regulation.
“The 3D structures of proteins are the keys to understand the mechanisms of life processes. Among more than 100 thousand solved protein structures among the world, 86% of them were determined with the synchrotron light and the protein crystallography technique. In recent years, six Nobel prizes were given to scientists who conduct their researches using this technique,” Yuch-Cheng Jean, a beamline scientist at the NSRRC, mentioned.
The Taiwan Photon Source, constructed with 7 billion TWD in Taiwan, is inaugurated on January 25, 2015. The NSRRC is commissioning one of the phase-I beamlines, Protein Microcrystallography Beamline, since November 2015. It is scheduled to be open to domestic and international users in September 2016.
Scientific Reports 5, 2015 (doi:10.1038/srep16441)
|
Distribution of Typhoid fever patients in the world (source: Centers for Disease Control and Prevention, USA) |
The structure of outer-membrane protein of Salmonella Typhi. A “D-cage” located at the entrance captures the antibiotics before secretion outside the pathogen through the channel. |
The new mechanism of the efflux pump.
The ST50 on the membrane surface of Salmonella Typhi works as a pump and results in drug resistance. |
The Protein Microcrystallography Endstation at the SPring-8 BL12B2
|
SPring-8 BL44XU: The Macromolecular Assemblies Beamline
|
- Current article
- New Protein Discovery Solves Myth of Bacterial Drug Resistance (Press Release)