Life Science
Exploration of the Mysteries of Life at the Molecular Level

Unparalleled Performance in the Analysis of Protein Structures
Life science research is an important component of basic science. In the life sciences, we explore the mechanisms of biological phenomena. Application of the knowledge obtained from life science research can lead to advancements in medicine, as well as help to solve food and environmental problems. All of these applications are expected to contribute to the improvement of people's lives and the development of the economy.
Biological bodies can be characterized as complex systems comprising numerous molecules, including proteins and DNA. At the same time, they contain multiple 3D layers of well-organized structures, and are capable of functioning in an orderly manner. To investigate such complex systems, we have to develop techniques that are optimized for making measurements at scales appropriate to various objects, ranging from atoms and molecules through intracellular organelles to cells and organs. It is also important to dynamically observe biological events in real time. In order to observe objects at such a broad scale, ranging from the whole body to the molecular level, the field of view for observation must also range from meters to sub-nanometers (Fig. 1). Synchrotron X-rays at SPring-8, which offer a wide field of view and high precision, are especially useful for visualizing biological systems through the interactions between X-rays and the electrons in the objects.
First of all, focusing attention on the molecular level, we find that proteins play a major role in biological phenomena. The unique 3D structure of each protein is responsible for the manifestation of its particular functions. Structural biology is the discipline that investigates the structures and functions of proteins. X-ray crystallography is the most promising technique for precisely determining these structures. The molecular structures of proteins can be determined by analyzing diffraction images obtained by subjecting crystallized proteins to X-rays (Fig. 2).
There are tens of million kinds of proteins, but their basic constituent structural components are believed to be less numerous, on the order of ten thousand. After the completion of the decoding of the human genome, the Protein 3000 Project was launched in 2002 as a national project in Japan. The aim of this project was to establish a research foundation to reveal the 3D structures and functions of proteins by strategically allocating resources. Structures of about 3,000 proteins, which account for one-third of the total, were targeted for investigation. This project promoted the establishment of appropriate intellectual property rights and technology transfer, with the goal of becoming the world leader in generating results and developing applications for these research findings. Since proteins are involved in the onset of many diseases, the exploration of their structures and functions is expected to accelerate the development of new drugs. This project is aimed at translating research outcomes into clinical advances that will benefit people's health and longevity over the long term. SPring-8 played a central role in this project, which was completed in 2006. More than 80% of protein structures registered to the international Protein Data Bank (PDB) are obtained from X-ray crystallography; and SPring-8 contributed to 65% of the structures submitted from Asia. The latter figure strongly emphasizes the importance of SPriong-8 in the analysis of protein structure.
The crystallization of proteins is indispensable in the analysis of the 3D structures of proteins. However, the crystallization itself remains challenging. It is even more difficult to obtain large protein crystals suitable for determining structures. It is especially hard to obtain good crystals for many membrane proteins and protein complexes, which play important roles in the understanding of biological phenomena and the development of new drug. Precise structural information can be obtained even from small protein crystals by using extremely brilliant synchrotron X-rays produced at SPring-8. These X-rays have been contributing to many breakthrough research projects by revealing the structures of proteins that are difficult to crystallize, such as rhodopsin, as well as the structures of calcium pumps, gap junction channels, and bacterial flagellar proteins (flagellins). Following on the successes of the Protein 3000 Project, a successor project was initiated: the Targeted Proteins Research Program, which aims to reveal the structures and functions of selected important proteins. To support this new project, we constructed the RIKEN Targeted Proteins beamline (BL32XU), which can produce narrow X-ray beams with a beam size of ~1 μm (10-6 m) in diameter (microbeams), to analyze even smaller crystals. BL32XU has been in full operation since May 2010. Hence, we expect further advancement in the structure analyses of proteins that have heretofore been difficult to analyze.
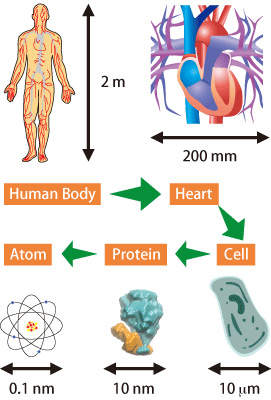
Fig. 1. Organisms and scales
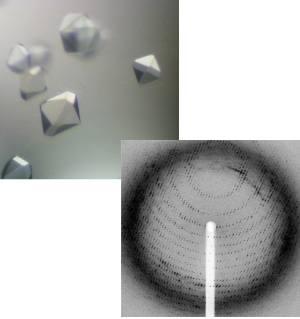
Fig. 2. Protein crystals (top) and diffraction image of proteins (bottom)
Visualizing Objects ranging from a Single Molecule to Organs
On the other hand, how can we investigate biological phenomena in real time? The crystal structures of proteins are static, but we can study their dynamic functions by applying the following technique: first, the movement of a protein is halted at several stages, in order to capture its structure at each stage; next, the captured structures are sequentially combined. Dynamic functions of calcium pumps have been described using this technique. Other features of proteins, which cannot be determined by crystal analysis alone, can also be elucidated by combining crystal analysis with other techniques, e.g., the combination of crystal analysis with electron diffraction; this technique has demonstrated its tremendous potential in the analyses of gap junction channels and flagella.
Direct observation of the behavior of proteins in solution is also an important way to examine their functions. X-ray solution scattering is a suitable technique for observing the dissociation and association of molecules as well as their macroscopic structural changes. Taking advantage of the features of this technique, we revealed the underlying mechanisms of the bacterial clock system by purifying several molecules out of the complex system and observing them in solution.
These techniques amplify the interactions of X-rays and electrons by treating multiple molecules collectively to produce a state that can easily be detected; thus, high-resolution images can be obtained. However, the description of the functions of each individual molecule is still difficult. To overcome this difficulty, new measurement techniques have been developed to directly observe the real-time functions of a single molecule by labeling it. The real-time movement of potassium channels has been observed by tracing the X-ray diffraction signals emitted from small gold crystals that are attached to proteins.
Additionally, the motion of molecules in the living bodies has been explored by using powerful X-rays at SPring-8. The functions of myosin, which is a protein responsible for myocardial contraction, were difficult to observe using existing techniques. At SPring-8, X-ray diffractions from myosin have been detected by transmitting X-rays through the hearts of live mice. This achievement can be applied to the diagnoses of cardiac dysfunction.
Transmission X-ray imaging is also indispensable beyond the cell level, in observations at the organ level. Refraction contrast imaging, synchrotron X-ray CT imaging, and micro CT imaging using X-ray microbeams can yield higher resolution images than those obtained from the conventional medical X-ray imaging commonly used in hospitals. Additionally, phase-contrast X-ray CT imaging, in which the wavefront distortion (phase contrast) is measured, can clearly reveal the structures of the rat brain. Furthermore, X-ray imaging of organs such as the hearts or lungs of live small animals, whose motions are rhythmic and extensive, can be enabled by synchronizing the timing of image capture with heartbeat or breathing. Real-time movement of the lungs of newborn rabbits has been observed using refraction contrast imaging. SPring-8 is the only facility in the world that enables such high-resolution measurement of the lungs.