Vesicular Transport Research Proposing a New Skin Whitening Mechanism
Mechanism of sunburn
When exposed to strong sunlight, our skin is burnt and becomes darker in color. This is explained as follows. Melanocytes in the epidermal basal layer produce black melanin pigments upon stimulation by the ultraviolet rays in sunlight, and the melanin pigments are transferred to keratinocytes in the skin, leading to the keratinization of cells (Fig. 1). Keratinization is an important mechanism that protects human bodies from harmful ultraviolet rays; however, many women dislike the skin darkening and blotches resulting from sunburn. With the aim of responding to the requests of such women, research on skin whitening to determine how to maintain a light complexion is being actively carried out.
One of the methods of maintaining a light complexion is to prevent melanocytes from synthesizing melanin pigments. Some substances that have this function have already been indentified and applied to skin-whitening cosmetics. In another method, the skin whitening effect may be obtained by preventing the synthesized melanin pigments from being transferred to keratinocytes. In 2004, Professor Mitsunori Fukuda of the Graduate School of Life Sciences, Tohoku University, clarified the mechanism of transport of melanin pigments inside melanocytes. The achievements of his research are drawing attention and are expected to lead to the development of new skin whiteners.
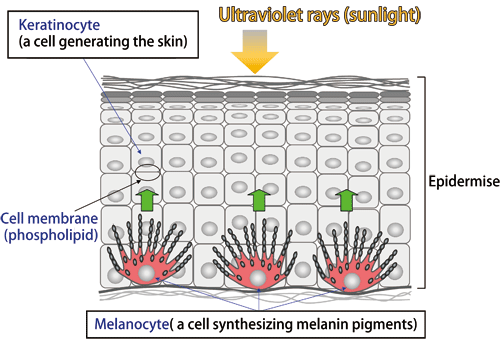
Fig.1 Structure of the skin
Melanin transport similar to transport by truck
According to Professor Fukuda, melanin transport, which is a complicated system involving various proteins, can be easily understood by comparing it to transport by trucks. He makes various analogies to help people understand the system (Fig. 2).
Melanosomes are the ‘parcels’ transported by ‘trucks.’ Melanosome is a granule that is composed of a phospholipid container called the vesicle*1 and melanin pigments encapsulated in it. To transport a parcel correctly to a keratinocyte, a tag indicating the destination of the parcel is necessary. Rab27A*2 functions as the tag, and two proteins, Slac2-a and Slp2-a, follow the indication of the tag.
Slac2-a, described as the driver, binds to the ‘parcel’ of Melanosome via the ‘tag’ of Rab27A. It also binds to myosin Va, a motor protein promoting the transfer of melanosomes in a cell. This is like a driver carrying a parcel and getting into the truck. The ‘truck’ of myosin Va runs on a ‘road’ of actin filaments in the cell.
When the truck approaches the ‘destination,’ i.e., the cell membrane of the melanocyte that faces the keratinocyte, Slp2-a starts working as a ‘deliverer,’ who gets off the truck to place the parcel at the entrance of the destination. Slp2-a attaches the melanosome to the cell membrane of the melanocyte so that the melanosome is transferred to the keratinocyte.
The details of melanin transport have been clarified up to this level, although the entire mechanism has not yet been clarified (Fig. 3). In addition to actin filaments, ‘roads’ of microtubules radially extend around the nucleus in a melanocyte. Melanosomes synthesized around a nucleus are transported along microtubules to the front of actin filaments. It is unclear how melanosomes are transported along microtubules. More basically, how melanin pigments are stored in melanosomes is unclear. Research on these issues is being carried out with the aim of clarifying the entire mechanism of melanin transport.
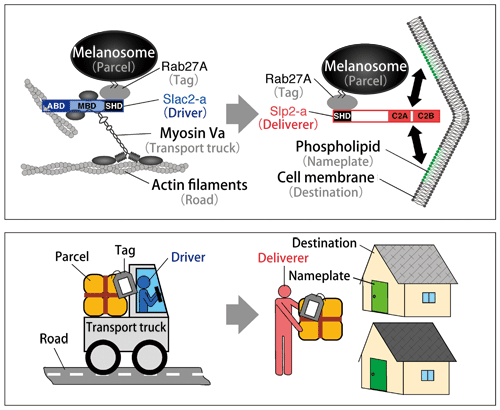
Fig.2 Truck transport model
Both a driver (Slac2-a) and a deliverer (Slp2-a) transport a parcel (melanosome) following the indication of a tag (Rab27A).
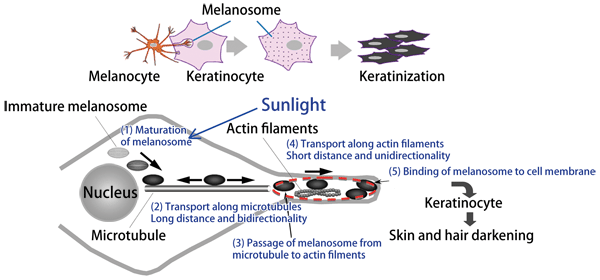
Fig.3 Mechanism of melanosome transport in melanocyte
Processes (1) and (2) have not been completely clarified yet. The mechanism in the region indicated by the red dotted circle is explained by the truck transport model in Fig. 2.
Long path to clarifying the mechanism
There is a reason that Professor Fukuda has successfully clarified the mechanism of melanin transport ahead of others. His specialized field is intercellular trafficking, which involves vesicles and includes neurotransmitter release. During the course of his research, he identified Slp2-a by chance (upper right in Fig. 2). Slp2-a has amino acid sequences used for binding to a cell membrane, i.e., C2A and C2B. Therefore, it is speculated that Slp2-a is related to the transport of some substances. When exploring an amino acid sequence that would provide a clue for clarifying the substance transported by Slp2-a, he identified the Scar homology domain (SHD) in Slp2-a, which is also identified in Slac2-a. Professor Fukuda considered that SHD binds to the tag of Rab, and examined all of the 60 known types of Rab regarding their binding to SHD with persistence. He finally found that only the 27th Rab proteins, i.e., Rab27A and Rab27B, bind to SHD.
Incidentally, a deficit in Rab27A genes causes failure in melanin transport, leading to the onset of Griscelli syndrome, in which the pigments in the patient’s skin and hair are lost. Slac2-a and Slp2-a may be related to melanin transport because they bind to Rab27A. It was observed that melanin transport is inhibited when parts of genes were deleted to prevent Slac2-a and Slp2-a from functioning (Fig. 4). Professor Fukuda constructed a truck transport model by considering the above experimental results.
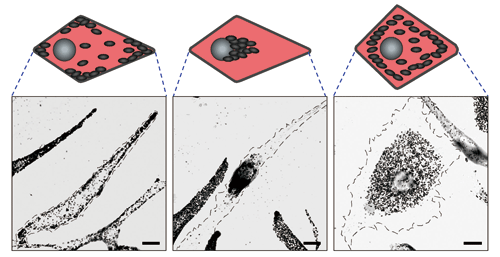
Fig.4 Bright-field microscopy images of melanocyte
Normal cell (left), Slac2-a-deficient cell (center), and Slp2-a-deficient cell (right). Each black granule represents a melanosome. When Slac2-a (driver) is deficient, melanosomes cannot be transported along actin filaments, ahead of microtubules, and remain around the nucleus. When Slp2-a (deliverer) is deficient, the melanosomes transported along the actin filaments cannot bind to the cell membrane and remain before the cell membrane.
Vesicular transport research drawing attention for the future
In 2008, Professor Fukuda analyzed and clarified the steric structures of the binding sites of Rab27B and Slac2-a using the Structural Biology Beamline I, BL41XU, at SPring-8 in cooperation with Shigeyuki Yokoyama, director of the Systems and Structural Biology Center at RIKEN (Fig. 5). Professor Fukuda, who had already examined amino acids related to the binding sites, said that he was pleased to observe the binding sites in the steric structures as he expected. However, he also said that he found a missing point unexpectedly, highlighting the significance of research for clarifying steric structures. At that time, Professor Soichi Wakatsuki of the High Energy Accelerator Research Organization clarified the steric structures of the binding sites of Rab27A and Slp2-a. Although Professor Fukuda encountered various difficulties, such as problems in obtaining stable proteins, the data on the structure they determined will greatly contribute to the exploration of new skin whiteners and development of drugs for preventing gray hair in the future.
Professor Fukuda welcomes the fact that his research achievements are being applied to the development of skin-whitening cosmetics and other drugs. According to him, vesicular transport is an interesting phenomenon because it occurs in various processes, such as neurotransmission and the secretion of hormones and digestive juices. Unless substances in cells are appropriately transported, various diseases may develop. He would like to widely study not only melanin transport but also other various types of vesicular transport. The clarification of the mechanism of melanin transport was the first step.
He talked about his future plan to clarify the functions of the tag protein Rab, which has rarely been studied, by taking advantage of his laboratory having all 60 types of Rab. He considers that research on vesicular transport will become more attractive.
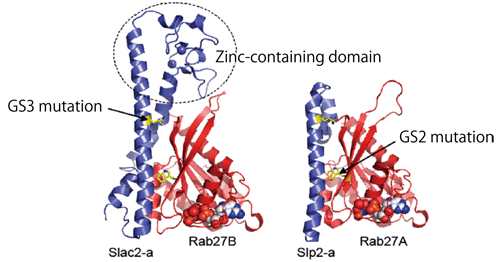
Fig.5 Steric structures of binding sites in Rab27B-Slac2-a complex (left) and Rab27A-Slp2-a complex (right)
It is clear which amino acids are involved in binding. It was difficult to synthesize a stable protein that can be used for structural analysis; therefore, Rab27A was partly replaced with Rab27B, which has a structure similar to that of Rab27A. This replacement is considered to cause no problem because the proteins that form a complex with Rab27A also bind to Rab27B.
(Right figure courtesy of Professor Soichi Wakatsuki, director of the Structural Biology Research Center at the High Energy Accelerator Research Organization, Inter-University Research Institute Corporation)
Really enjoying the nature of Aobayama
 |
He took a picture of a horse fly in a park when he played with his two sons.
|
“Five years ago, my son entered elementary school and I bought a cellular phone to keep in contact with him, but I usually use it as a camera rather than as a phone,” says Professor Fukuda. He has snapped about 2000 pictures of living things, including butterflies, dragonflies, frogs, and plants. He said, “Recently, I have become addicted to taking photos of shield bugs; the attraction of these plain bugs is not easily understood by other people.” He is an insect connoisseur. This may be why he entered university to study ecology. His photos are uploaded on his homepage, so please visit the site. He sometimes collects pupae and lets his son take them to school, or he brings them to his laboratory. No matter how busy he is in his research, Professor Fukuda never forgets to enjoy the changes of the seasons in Aobayama, where the campus of Tohoku University is located.
Professor Fukuda’s mobile photo studio is available at
http://www.ac.cyberhome.ne.jp/‾fukuda/photo.htm.
Interview and original text by Akiko Ikeda (Sci-Tech Communications)
Glossary
*1 Vesicle
Vesicles are bladderlike cavities surrounded by a membrane in a cell. Vesicles are used to synthesize and store substances in a cell and transport them between the inside and outside of the cell.
*2 Rab27A
Rab is a protein that mainly functions as a ‘tag’ during intercellular vesicular transport. Approximately 60 types of Rab have been identified in humans; among them, the 27th protein, Rab27A, is involved in melanosome transport. Rab27A was partly replaced with Rab27B, which has a function similar to that of Rab27A, to obtain stable proteins for structural analysis.
This article was written following an interview with Professor Mitsunori Fukuda at the Laboratory of Membrane Trafficking Mechanisms, Developmental Biology and Neuroscience, Graduate School of Life Sciences, Tohoku University.