Do Bacteria and DNA Adsorb Rare Earth Elements? Opening the Door to Application from Atomic-Level Viewpoint
Rare earth elements, a key for 21st century technology and industry
Rare earth elements (REEs) such as neodymium (Nd), which is a material required for producing powerful magnets that are essential for PC hard disks, CD players, and cellular phones, and cerium (Ce), which is used as an abrasive and phosphor in manufacturing liquid crystal panels, are very important for the development of 21st century technology and industry.
REEs are a group of 17 elements including scandium (Sc) and yttrium (Y) in the third column from the left of the periodic table, and 15 elements from lanthanum (La) to lutetium (Lu) (collectively called lanthanides), which should be placed below Y but are usually shown separately at the bottom of the table because of space limitations (Fig. 1). Their atomic structures, particularly the unique electron configuration and behavior, are the cause of the strong magnetic force and luminosity of these elements.
Fig. 1 Seventeen REEs (indicated in yellow)
Major difficulties in separation and collection
One of the challenges in the application of REEs is the difficulty of separation and collection. Because some types of REEs are in a mixture with ores in nature, it is necessary to separate the desired elements from the mixture to utilize REEs. Generally, substances are separated by taking advantage of the differences in chemical properties such as melting and boiling points. However, because the chemical properties of REEs are similar, the separation of these elements is difficult.
The currently used separation method is the solvent extraction method. In this method, the solution of ores containing REEs is mixed with an organic solvent, and the REEs are once extracted in the organic phase and then reextracted in the aqueous phase. However, this method is too complicated and also accompanied by concerns about the adverse effects on the environment because of the use of organic solvents.
In addition, since China accounts for 97% of the global production of REEs as of 2009, it is important to collect REEs from used products to ensure a stable supply. However, because the collection of REEs requires the same technology as that required in their separation, Japan presently sends used products to China to collect REEs at a low cost. Under such circumstances, it is desired to develop a technology that enables the separation and collection of REEs in Japan.
Microorganisms in the kitchen have adsorption ability
Professor Yoshio Takahashi at Hiroshima University published his groundbreaking research results suggesting that bacteria adsorb REEs, which will lead to the solution of the above issues.
“It has been known that bacteria adsorb various elements, and research has been conducted mainly in the environmental field. Bacteria adsorb harmful substances such as lead and cadmium,” says Professor Takahashi.
Professor Takahashi thought that bacteria may also adsorb REEs. Through research, he found that bacteria adsorb REEs at an enrichment rate approximately 100,000-fold that in the surrounding solution. Although there are various bacterial types, very common bacteria, such as those living in the kitchen sink, show this degree of adsorption.
Interestingly, heavier REEs with high atomic number are adsorbed on bacterial cells at a higher adsorption rate than those with low atomic number. This finding will be beneficial because the separation and collection become easier as the adsorption rate increases and the price is higher for heavier REEs (Fig. 2).
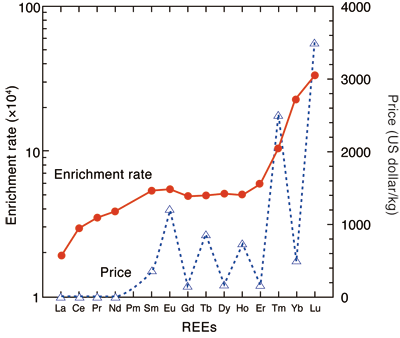
Fig. 2 Enrichment rate of REEs in bacteria compared with aqueous solution and price of REEs
(Enrichment rate: ratio of concentration of REEs in bacteria to that in diluted aqueous solution. The prices of the REEs are cited from Mineral Yearbook 2008.)
Clarification of adsorption mechanism at atomic level
Professor Takahashi not only found that bacteria adsorb REEs but also attempted to clarify the adsorption mechanism at the atomic level.
He conducted experiments using the extended X-ray absorption fine structure (EXAFS) method at the BL01B1 beamline of SPring-8 and the beamline of the High Energy Accelerator Research Organization. The EXAFS method is an experimental technique for measuring the level of X-ray absorption by a specific element (type of atom) in a substance as an absorption spectrum while changing the energy of the X-rays irradiated on the substance. The X-ray absorption spectrum differs depending on the type of atom being measured and also changes depending on the type and location of the surrounding atoms. Thus, the location and type of atoms in a substance can be determined by analyzing the obtained absorption spectra in detail. It was considered that REEs (in the cationic state) are adsorbed on either anionic carboxyl or phosphate groups, which exist in large numbers in the bacterial cell walls. Through his experiment, the phosphate groups were found to adsorb REEs (Figs. 3 and 4).
The phosphate groups are present in DNA, which contains genetic information. A study showed that DNA, similarly to bacteria, has the ability to absorb REEs. DNA is abundant in fish milts, and it is more suitable for practical use than bacteria. Professor Takahashi is now applying for a patent for the separation and collection of REEs by the solvent extraction method using a DNA-immobilized cellulose instead of an organic solvent. This method will enable the easier separation and collection of REEs without adverse effects on the environment unlike the method of using organic solvents.
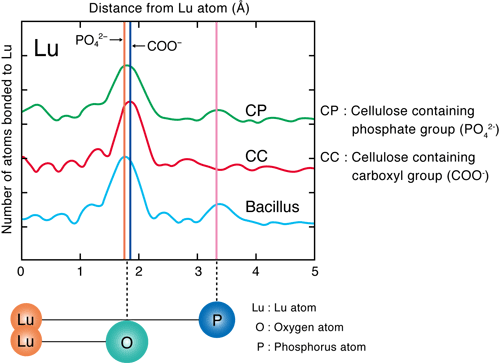
Fig. 3 Results of EXAFS analysis for Lu adsorption by bacterial cells (Bacillus)
The graph shows the elements existing in large numbers near Lu (the heaviest element among the REEs) in terms of the distance from the Lu atom. The graph for Bacillus closely resembles that for cellulose containing phosphate groups (cellulose phosphate; CP), indicating that the REEs are more strongly adsorbed on the phosphate groups (PO42-) than on the carboxyl groups (COO-) on the bacterial cell walls. (1 Å is 10-10 m)
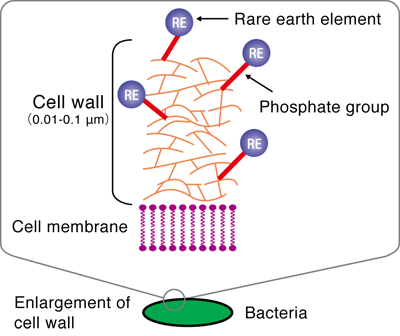
Fig. 4 Schematic of enrichment of rare earth (RE) on bacterial cell wall
A geochemical view is the origin of his idea
Professor Takahashi started his research without the intention of developing a new method of separation and collection of REEs. His specialty is in the field of geochemistry, which involves the study of elements existing within the earth, their states and function since the birth of the earth some 4.5 billion years ago. REEs are considered an important subject of research in this field.
“Measuring the percentage of REEs contained in a stone, we can determine whether the stone came from, for example, the deep mantle of the earth. I heard that bacteria have the ability to adsorb substances and tried to examine this ability in our research. Through such research, I found the possibility of applying this ability in industry as a new method of separating and collecting REEs,” says Professor Takahashi.
Research on the adsorption of REEs by bacteria had not progressed before Professor Takahashi started his research. Although the adsorption by bacteria was studied as a method of eliminating harmful substances, REEs were not the subject of such studies because they do not cause much harm to human health. It was from the unique viewpoint of geochemistry that enabled Professor Takahashi to focus on the adsorption of REEs by bacteria.
The clarification of the adsorption mechanism at the atomic level is also a geochemical approach. If the original target had been the solution of environmental problems or industrial applications, research on REEs would have been directed toward these practical applications after the phenomenon of the adsorption of REEs by bacteria was found. However, geochemistry involves the study of a very large object, the Earth, at the atomic level. Professor Takahashi, having acquired such a research perspective, was more interested in the clarification of the mechanism than in practical applications.
Bridge to applications based on atomic-level clarification
Professor Takahashi stresses that the important point of his achievements is the atomic-level clarification.
“The clarification of the mechanism of REE adsorption by bacteria generated the possibilities of new separation and collection methods using biologically relevant substances other than bacteria. The atomic-level data obtained from the microviewpoint provides a bridge from a macrophenomenon to the next applications of this phenomenon,” says Professor Takahashi.
He named such a research approach as atomic environmental geochemistry and is trying to foster his research. “Originally, I was interested in environmental problems and studied ozone depletion caused by chlorofluorocarbon when I was a student. Then my interest shifted to geochemistry. With time, my desire to do something useful for society through the atomic-level approach to environmental problems became stronger. Because X-ray experiment facilities such as SPring-8 are essential for atomic-level research, they will become more important in the future.”
In addition to this research on REEs, various research projects, such as the investigation of harmful substances contained in house dust and the study of microorganisms on the primitive earth through the observation of the seafloor hydrothermal systems, are being carried out in his laboratory. We would like to see how the viewpoint of atomic environmental geochemistry will promote the progress of such research activities and pave the way toward solving environmental problems.
Column: Students are my treasure
Soon after Professor Takahashi moved to Hiroshima University, he met a brilliant student who was assigned to his laboratory. When Professor Takahashi explained to the student the essence of the research he wanted to carry out and his research purpose, the student grasped those points and advanced his research, developing it to a much further extent than Professor Takahashi expected. Through this experience, Professor Takahashi came to believe in and carefully foster his students' abilities.
“If we set a defined purpose and trust students, they will carry out their research well. Some students need more time than others, but we have to wait without being impatient. I think what is most important for faculty staff is to really trust their students. Now I'm able to work on various subjects because brilliant students are playing active roles in my research projects. These students are my treasure,” says Professor Takahashi.
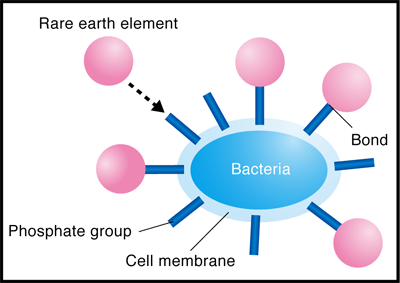 |
Image on cover page |
Interview and original text by Keiji Toeda
This article was written following an interview with Professor Yoshio Takahashi at the Graduate School of Science, Hiroshima University.