Clarification of Self-Assembly Mechanism of Organogelation at Molecular Level(Press Release)
- Release Date
- 29 Sep, 2010
- BL03XU (Advanced Softmaterial)
- BL40B2 (Structural Biology II)
The University of Kitakyushu
Japan Synchrotron Radiation Research Institute
|
A joint research team consisting of scientists from the University of Kitakyushu and Japan Synchrotron Radiation Research Institute clarified the molecular structure of a solvent in a gel state gelated by an organogelator (low-molecular-weight compound) using the high-brilliance synchrotoron radiation at SPring-8. The organogelator is capable of gelating the entire solvent merely by adding an amount equal to approximately 1% of the solvent. It is expected that the application of this compound to solidify crude oils will prevent environmental pollution due to oil spillage in the sea. The achievement in this study will greatly contribute to the development (molecular design) of organogelators for solidifying crude oils. Publication: |
<Background of research>
When viewing living organisms at a molecular level, it is understood that several molecules are combined to form regular structures that play functional roles. In addition, vital functions are maintained through complicated and cooperative interactions between several molecular groups. The chemical bonds forming each molecule are covalent, and are a main research target in chemistry. However, the covalent bond plays only a limited role in biological functions. The regular molecular arrangements and high-order functions of biological aggregates are realized through the combined actions of weak bonding forces other than that of covalent bonds. Supermolecular chemistry deals with the phenomena due to the cooperative functioning of such weak bonding forces. Supermolecular chemistry deals with low- and high-molecular-weight compounds; the phenomena exhibited by the aggregates of such compounds with sizes ranging from 20 nm to 1 μm or less are the targets of supermolecular chemistry. The aggregates are not structured in a regular pattern as in the crystal structure of the low-molecular-weight compounds; rather, they are hierarchical and fluctuate temporally and spatially. Organic-synthesis chemists have played a leading role in the research of supermolecular chemistry. However, the number of molecules with actually observed structures that were imagined in the molecular design phase is limited. Small-angle X-ray scattering using synchrotron radiation including intense X-rays is effective for the structural analysis of such molecules.
One of the important research targets of supermolecular chemistry is the development of the organogelator, which is capable of gelating the entire solvent merely by adding a low-molecular-weight compound in an amount equal to approximately 1% of the solvent. One familiar example is the agent used to solidify used cooking oil. The application of organogelators to solidify crude oils has been attracting attention. Oil spills in oceans, as exemplified by the Gulf of Mexico oil spill, create serious environmental pollution. If it becomes possible to solidify and store crude oils using an organogelator to prevent oil spills in the case of tanker accidents and to immediately return the gel state to the fluid state upon applying appropriate stimulation to the gel so that the oils can flow through pipes, the use of such an organogelator will be particularly effective as a green chemistry that can protect the environment. To this end, it is necessary to develop a low-cost material that can change the crude oils into a gel state with the addition of only a small amount and return the gel to a low-viscosity fluid upon the application of appropriate external stimulation. Therefore, the clarification of the mechanism underlying the gelation and molecular design should be promoted through accumulation of fundamental research.
<Research contents and achievements>
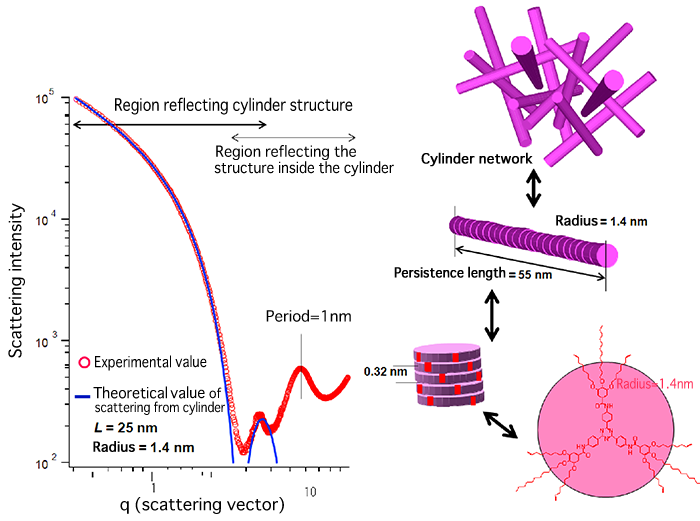
In this study, the mechanism underlying the gelation of a solvent by an organogelator was clarified through the examination of (1) the stacking of each molecule, (2) the rodlike aggregates formed by the self-assembly of molecules, and (3) the hierarchical structure consisting of a cylinder network of aggregates by small-angle X-ray scattering (SAXS)*1 and wide-angle X-ray scattering (WAXS)*2 using BL40B2 Structural Biology II at SPring-8. Molecules stack spirally at an interval of 0.32 nm. The segment length,*3 an indicator of the rod flexibility, is as long as 55 nm, indicating that the aggregates behave like rods and that gel is formed by the cylinder network of these rods (Fig. 1). This achievement could not have been obtained without the high-brilliance synchrotron radiation at SPring-8 and the vacuum chamber that can minimize the measurement noise (in a vacuum chamber, a target sample in a sealed container is placed in vacuum and X-rays are irradiated for measurement) developed jointly by Dr. Masunaga of JASRI and a research group led by Professor Sakurai of the University of Kitakyushu. This study was especially novel in that the hierarchical structure with sizes ranging from the atomic order of 0.1 to 1,000 nm of the organogelator was analyzed in detail. The image of the atomic structure of the gelled organic solvent octane graced the cover of the October issue of Polymer Journal published by Nature Publishing Group (Fig. 2).
<Future prospects>
In this study, the molecular structure of an entire solvent in a gel state achieved using an organogelator was clarified, and such a solvent is highly promising as a measure against environmental pollution due to oil spills in oceans. It is expected that organogelators with which the solidification and fluidization of oils can be controlled will be developed through the accumulation of fundamental research using this achievement as a basis, to realize a safe and secure society without the worry of sea pollution due to oil spills.
The BL03XU Advanced Softmaterial Beamline supported by the Advanced Softmaterial Beamline Consortium*4 began operations in April 2010 and has been used for the research of soft materials. Professor Sakurai is the chairman of the steering committee of the consortium and his coresearcher, Dr. Masunaga, is in charge of the BL03XU beamline. In this study, only static measurement was carried out; it is expected that the dynamic characteristics of the organogelator will be clarified using the high-brilliance synchrotron radiation emitted from the BL03XU undulator,*5 which has the highest performance as a beamline dedicated to soft material analysis.
<Figure>

Copyright © 2010, Nature Publishing Group
Fig. 2 Image of atomic structure of gelated organic solvent,
octane, presented on the cover of Polymer Journal
<Glossary>
*1 Small-angle X-ray scattering (SAXS)
SAXS is a technique for obtaining structural information of a material by measuring the X-rays with a small scattering angle (generally several degrees or smaller) among the X-rays irradiated and scattered from a material. With SAXS, the analysis of microphase-separation structures of high-molecular-weight compounds and the regular inner structures of microparticles, liquid crystals, and alloys, with sizes ranging from 2 to 300 nm, is possible.
*2 Wide-angle X-ray scattering (WAXS)
WAXS is a technique for analyzing angstrom-order characteristics (an angstrom is 0.1 nanometer), such as atomic arrangement in crystals, by measuring the X-rays with a large scattering angle (generally 10o or larger) among the X-rays irradiated and scattered from a material.
*3 Segment length
When gel is considered to form a structure consisting of hard rods and flexible connections, the segment length is the length of the hard rods.
*4 Advanced Softmaterial Beamline Consortium
The Advanced Softmaterial Beamline Consortium consists of scientists from 19 corporate entities (including related research and business institutions), universities, foundations, and independent administrative institutions and manages the operation of the dedicated beamline BL03XU at SPring-8. Please visit the website of the consortium at http://fsbl.spring8.or.jp/index.html, for detailed information.
*5 Undulator
An undulator is a light source in which permanent magnets with opposite poles are aligned alternately in upper and lower series. Synchrotron radiation is generated from electron beams meandering between the two series of permanent magnets.
For more information, please contact: |
- Previous Article
- Multistep Ionization of Ar Atoms by Extreme-Ultraviolet Free Electron Laser (Press Release)
- Current article
- Clarification of Self-Assembly Mechanism of Organogelation at Molecular Level(Press Release)