Successful Development of Method Capable of Simply and Quickly Analyzing Characteristics of Membrane Proteins (Press Release)
- Release Date
- 25 Feb, 2011
- High-throughput
RIKEN
Key research findings
○ Realizing high-throughput structural analysis of membrane proteins, which has conventionally taken much time and effort
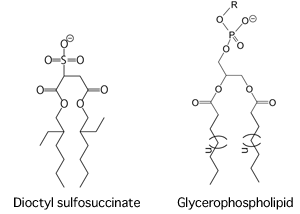
○ Achieving high-resolution electrophoretic separation of membrane proteins using dioctyl sulfosuccinate while maintaining their native structure
○ Hoping the established method will be an effective tool for studies on membrane proteins including analysis of their three-dimensional structure
|
RIKEN (President, Ryoji Noyori) has developed a new fluorescence detectable native polyacrylamide gel electrophoresis (FN-PAGE) method that enables the high-throughput screening of samples used for the structural analysis of membrane proteins and the determination of their preparation conditions. This was achieved by Atsuko Yamashita (Team Leader), Makoto Ihara (Research Scientist), and Noriko Matsuura (Research Assistant) of the Molecular Signaling Research Team, Structural Physiology Research Group, RIKEN SPring-8 Center (Director, Tetsuya Ishikawa). Membrane proteins are embedded in biological membranes, such as cell membranes, account for approximately 30% of the types of protein in living organisms, and are involved in many important vital functions. As many membrane proteins are used as target molecules in medicine, approximately half of commercially available medicines target membrane proteins. To understand vital functions and efficiently design medicines that work only on target molecules, it is very important to clarify the three-dimensional structure of membrane proteins. However, the structural analysis of membrane proteins has not satisfactorily progressed thus far because the physiological structure of membrane proteins are easily damaged or degenerate during the preparation of samples used for structural analysis. The research team focused on the native PAGE method※1 as a means of screening samples at high throughput in terms of their characteristics, such as whether the native structure of prepared membrane protein samples is properly maintained, and explored the conditions for this method. The research team succeeded in finding a condition for achieving good electrophoretic separation that can yield sharp electrophoretic bands and during which the structure of the membrane proteins is maintained, that is, the use of dioctyl sulfosuccinate, a sulfosuccinate anionic surfactant, as the electrophoretic reagent. When membrane proteins linked to green fluorescent proteins (GFPs) are used in this method, the detection of fluorescence from the GFPs becomes possible, enabling the rapid analysis of the characteristics of a sample without the need for purification. The established method is effective for screening samples used for structural analysis using SPring-8 beamlines, including X-ray crystallography, at a high throughput and for determining their preparation conditions. This method is also expected to accelerate not only the analysis of three-dimensional structures but also various studies on membrane proteins. This research was supported by the "Target Proteins Research Program" promoted by the Ministry of Education, Culture, Sports, Science and Technology, and the results were published in the American scientific journal Analytical Biochemistry. (Publication) |
<<Glossary>>
*1 Native PAGE method
In the native PAGE method, native proteins that maintain their original physiological structure migrate. In the conventional native PAGE method, electrophoretic separation does not always accurately correspond to the molecular weight of the proteins because the migration behavior of the proteins in a gel is affected by the shape and surface electric charges of the proteins as well as their molecular weight.
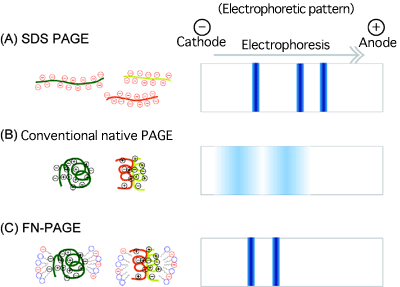
<<Figure>>
(A) Sodium dodecyl sulfate (SDS)-PAGE
Proteins denatured by SDS are subjected to electrophoresis. Sharp electrophoretic bands showing the mobility corresponding to the molecular weight of the proteins can be obtained because SDS cancels the electric charges of the proteins. However, the molecular weight of the proteins with the native physiological structure cannot be determined.
(B) Conventional native PAGE
Denaturants such as SDS are not used to maintain the native physiological structure of the proteins during electrophoresis. Because the electrophoretic migration of the proteins is affected by their electric charges, electrophoretic bands are wide, and the mobility does not correspond to the molecular weight of the proteins in many cases.
(C) Improved FN-PAGE
The physiological structure of the proteins is maintained during electrophoresis. In addition, the electric charges of the proteins are cancelled by the anionic surfactant dioctyl sulfosuccinate added during electrophoresis, yielding sharp electrophoretic bands. The mobility corresponds to the molecular weight of the proteins. Therefore, the molecular weight of the proteins, in which the native physiological structure is maintained, can be determined.
a principal constituent of living membranes
In the chemical structure of glycerophospholipid, R represents choline, ethanolamine, serine, inositol, or others, and n is usually 10-12 (the number of carbon chains in fatty acids is usually 16-18; sometimes they include unsaturated bonds). Dioctyl sulfosuccinate and glycerophospholipid commonly have a polar head group (sulfur group for the former and various alcohol phosphate groups for the latter) and two hydrophobic tails.
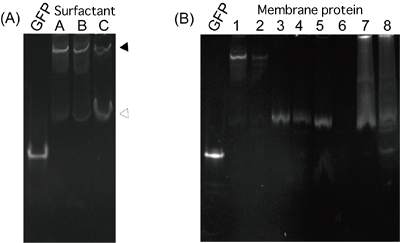
(A) GFP fusion protein with trimer membrane protein A physiological structure, trimer (indicated by the closed triangle), is broken by a soluble surfactant, and a monomer (indicated by the open triangle) is produced. In the case of this membrane protein, the monomer percentage for surfactant C is higher than those for surfactants A and B, indicating that surfactant C makes the sample protein unstable.
(B) GFP fusion protein with various membrane proteins
Membrane proteins 1-4, with good characteristics, that have already been successfully analyzed by X-ray crystallography show sharp bands. However, the crystallization of membrane proteins 5-8 has not yet been successful; no good expression is achieved and no electrophoretic band is obtained for membrane protein 6, and the electrophoretic bands are wide for membrane proteins 7 and 8. Whether the characteristics are good or poor can be determined by the difference in migration pattern.
|
For more information, please contact: |
- Previous Article
- Clarification of Structure and Molecular Mechanism of Reductase that Eliminates Misfolded Proteins in Cells (Press Release)
- Current article
- Successful Development of Method Capable of Simply and Quickly Analyzing Characteristics of Membrane Proteins (Press Release)