Clarification of Structure of Giant Motor Protein Functioning in Cells (Press Release)
- Release Date
- 23 May, 2011
- BL44XU (Macromolecular Assemblies)
Osaka University
|
A research group led by Associate Professor Takahide Kon and Professor Genji Kurisu of the Institute of Protein Research, Osaka University has analyzed the entire structure of the dynein motor with motor functions and partially clarified the mechanism of this giant molecular motor that “walks” in a cell using long stalk-like structures. In cells constituting our body, a group of proteins called molecular motors activates various cell movements by converting chemical energy into that used for mechanical movement. Among the major molecular motors, only the “dynein motor,” which plays an important role in the transport of intracellular substances and in ciliary and flagellar movements, remains to be clarified of its entire three-dimensional structure because of its huge molecular size. The dynein motor plays essential roles in the transport of intracellular substances and in human cell division. Also, it is the only molecular motor that activates the rippling movements of cilia and flagella such as those of sperm tails. The achievements of this research group are significant in that they clarified the basic mechanism of the activation of these important cell movements within the body. The data of the X-ray crystallography were obtained using the Macromolecular Assemblies Beamline, BL44XU, of SPring-8. The achievements were published online in the British scientific journal Nature Struct. Mol. Biol. on 22 May 2011 prior to publication in the printed version. Publication: |
In cells constituting our body, a group of proteins called molecular motors activates various cell movements by converting chemical energy into that used for mechanical movement. The myosin motor triggers muscle contraction by its sliding movements on the fibrous filament called actin. Two other molecular motors called the kinesin and dynein motors transport various intracellular substances over a long distance by their translatory movements on microtubule rails in the cells. Among these three major molecular motors, the dynein motor is the only molecular motor that remains to be clarified of its entire three-dimensional structure because of its huge molecular size and complicated structure, making research difficult.
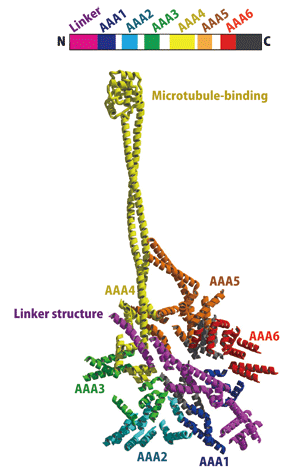
The research group clarified the three-dimensional structure of the dynein motor for the first time in the world by crystallizing a perfectly functioning dynein motor of cellular slime molds (Fig. 1). As a result, they found that the dynein motor has two long stalks protruding from the AAA+ ring, which is the site for ATP hydrolysis, and that the dynein motor moves on microtubules while changing the structure of its two stalks. Also, they observed a lever-arm structure (linker structure) for force generation spanning the AAA+ ring, indicating that the lever arm generates force by its swinging movements. These structural characteristics are entirely different from the conventional characteristics of myosin and kinesin. The dynein motor is the third molecular motor that moves on rails with a newly found motion mechanism.
The dynein motor plays an essential role in the transport of intracellular substances and in human cell division. Also, it is the only molecular motor that activates the rippling movements of cilia and flagella such as those of sperm tails. Their achievements are significant in that they clarified the basic mechanism of the activation of these important cell movements within the body.
<<Figures>>
The linker structure (indicated in purple) spans the AAA+ ring, and the long stalk-like structures (indicated in yellow and orange) protrude from the site. The small ball-shaped tip of the yellow stalk is bonded to a rail called a microtubule.
|
For more information, please contact: |
- Previous Article
- World’s First Clarification of Mechanism of Protein Export Enhanced by Effects of Ions (Press Release )
- Current article
- Clarification of Structure of Giant Motor Protein Functioning in Cells (Press Release)