Mechanism Underlying Removal of “Repressive Mark” on Genes by Ubiquitously Transcribed Tetratricopeptide Repeat, X Chromosome (UTX)(Press Release )
- Release Date
- 14 Oct, 2011
- BL41XU (Structural Biology I)
RIKEN
Key research achievements
• Ubiquitously transcribed tetratricopeptide repeat, X chromosome (UTX) recognizes details of histones and distinguishes a repressive mark from other marks.
• The unique structure of UTX plays a significant role in distinguishing the repressive mark from other marks.
• The findings provide an important step toward the development of UTX inhibitors that will lead to the regulation of cell differentiation.
RIKEN (President, Ryoji Noyori) has, for the first time ever, determined the three-dimensional structure of UTX, a protein that removes a repressive mark bound to genes, and clarified how UTX specifically distinguishes the repressive mark from marks of other functions and removes only the repressive mark. This was achieved by Shigeyuki Yokoyama (Director) and Toru Sengoku (Research Scientist) of RIKEN Systems and Structural Biology Center. In higher organisms, the activities of genes are regulated not by the changes in DNA base sequences but by various chemical marks bound to the genes. DNA closely binds to a group of proteins called histones.*1 The methylation of a certain site (the 27th lysine residue; lysine 27) of histone H3, among several types of histone, is a chemical mark of repression, which silences the activities of genes. On the other hand, the methylation of other sites of histone H3 indicates a different function. UTX removes only the methyl group bound to lysine 27, which has an important role in turning on genes in certain cells that should work at the right time. Thus, UTX is essential for cell differentiation in many animals including humans and also acts as a tumor-suppressing protein. The research team has succeeded in determining the three-dimensional structure of UTX bound to histone H3 by X-ray crystallography*2 at SPring-8. By biochemical analysis on the basis of the determined three-dimensional structure, the team found that UTX is extensively bound to histone H3 and has a unique structure for recognizing not only the characteristics of sites around lysine 27 but also those of a distant domain. With such a structure, UTX can accurately remove only the methyl group bound to lysine 27. These findings will be useful in understanding cell differentiation and will also contribute to the development of drugs for the artificial regulation of cell differentiation. This study was carried out as part of the "Targeted Proteins Research Program" and the "National BioResource Project (Drug-Discovery Supporting Technology Platform)" supported by the Ministry of Education, Culture, Sports, Science and Technology. The achievements were published online in the American scientific journal Genes and Development on 14 October 2011 prior to publication in the printed version on 1 November 2011. Publication: |
<<Glossary>>
*1 Histones
Histones are a group of proteins that bind to genomic DNA and are a major component in the structure of chromosomes in eukaryotes. There are five types of histone, namely, histones H1, H2A, H2B, H3, and H4. They undergo various chemical modifications such as methylation, acetylation, and phosphorylation at various sites that regulate the activities of genes.
*2 X-ray crystallography
X-ray crystallography is a method of examining the internal structure of a substance by forming crystals, irradiating the crystals with X-ray, and analyzing the obtained diffraction data. It is one of the most effective methods to clarify in detail the structure of proteins at an atomic-level resolution.
<<Figures>>
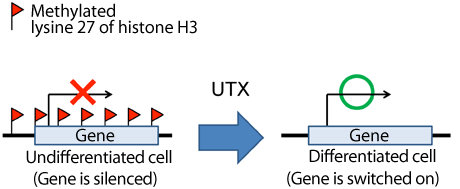
The methylated lysine 27 of histone H3 acts as a repressive mark. UTX removes the methyl group bound to lysine 27 and switches on the gene to function in certain cells at the right time.
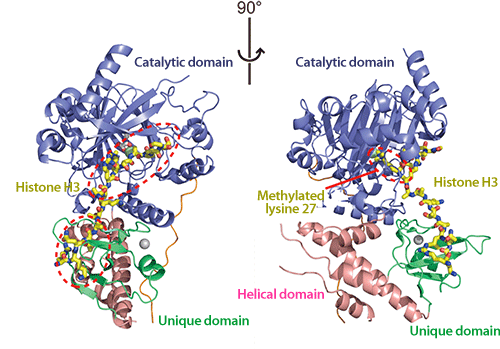
The two images show the same structure rotated 90 degrees.
(Left) UTX and histone H3 are bound to each other at the two areas encircled by the red dotted lines.
(Right) The methylated lysine 27 (encircled by the red dotted line) is bound to the catalytic domain. The methyl group bound to lysine 27 is removed here.
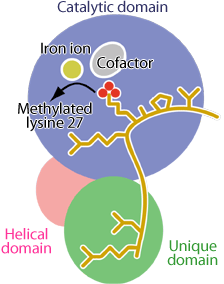
Both the catalytic domain (blue) and the unique domain (green) fit histone H3 (yellow) as in the lock-and-key model. The helical domain (pink) bridges the catalytic and unique domains.
|
For more information, please contact: |
- Previous Article
- Clarifying Three-Dimensional Structure of Adenomatous Polyposis Coli-Sam68 Complex Involved in Development of Colon Cancer (Press Release)
- Current article
- Mechanism Underlying Removal of “Repressive Mark” on Genes by Ubiquitously Transcribed Tetratricopeptide Repeat, X Chromosome (UTX)(Press Release )