Clarifying the Bonding State of Aluminum Storing a Significant Amount of Hydrogen Using Synchrotron Radiation (Press Release)
- Release Date
- 20 Oct, 2011
- BL27SU (Soft X-ray Photochemistry)
Japan Atomic Energy Agency
Japan Synchrotron Radiation Research Institute
Key research findings
• Clarifying the bonding state of hydrogen and aluminum, which is the key to improving the performance of hydrogen-storage materials, was achieved through experiments that can only be performed using synchrotron radiation.
• Directions were established for improving the performance of aluminum-based hydrogen-storage materials.
A research group of Japan Atomic Energy Agency (JAEA; Atsuyuki Suzuki, President), in collaboration with Japan Synchrotron Radiation Research Institute (JASRI; Tetsuhisa Shirakawa, President), carried out synchrotron radiation spectroscopy on aluminum hydrides, which are attracting attention as hydrogen-storage materials, and clarified that hydrogen and aluminum atoms are bonded by covalent bonds formed by shared electrons. Hydrogen is attracting attention as an ultimate source of clean energy. The key to utilizing hydrogen as an energy source is to develop materials in which a large amount of hydrogen can be stored and for which the hydrogen storage and release processes can be regulated under pressures and temperatures close to those of atmospheric conditions. Although aluminum hydrides are lightweight and can store a large amount of hydrogen, a high temperature and pressure are required for the hydrogen storage and release processes. To solve this problem, it is essential to understand the bonding state of aluminum and hydrogen atoms, which has remained unclarified until now. In this study, the research group determined the electronic states of aluminum and aluminum hydride by soft X-ray spectroscopy at SPring-8 and examined the difference in the electronic states before and after the storage of hydrogen. Also, comparing the experimental results with the theoretically calculated electronic state, it was found that a covalent bond is formed between the aluminum and hydrogen atoms. These results contradicted the theoretical prediction that these atoms would be ionically bonded. The clarification of the bonding state of hydrogen and aluminum atoms will not only contribute to understanding the hydrogen-storage and release processes of aluminum hydrides but also provide directions for the design of new hydrogen-storage materials based on lightweight and inexpensive aluminum. The bonding state of aluminium hydride was elucidated through joint research by Yukiharu Takeda (Senior Scientist), Yuji Saitoh (Senior Scientist), Hiroshi Yamagami (Visiting Scientist, currently Kyoto Sangyo University), Hiroyuki Saitoh (Senior Scientist), Akihiko Machida (Senior Scientist), and Katsutoshi Aoki (Specific Proposal Coordinator) of JAEA; and Takayuki Muro (Senior Scientist), Yukako Kato (Postdoctoral Fellow, currently a research scientist of the National Institute of Advanced Industrial Science and Technology), and Toyohiko Kinoshita (Chief Scientist) of JASRI. This study was commissioned by the New Energy and Industrial Technology Development Organization (NEDO) as part of the project "Experimental Elucidation of Hydrogen-Material Interactions" of Advanced Fundamental Research on Hydrogen-Storage Materials and was also accepted by JASRI as a research project utilizing SPring-8. The results were published online in the journal of the American Physical Society Physical Review B Brief Report on 10 October 2011. Publication: |
<<Figures>>
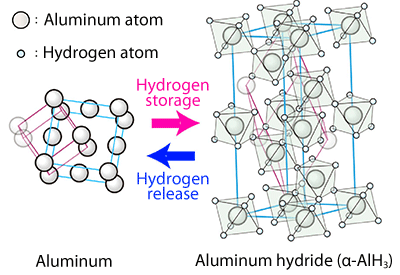
The structure changes significantly upon the storage and release of hydrogen. While aluminum has a face-centered structure, aluminum hydride has an octahedral structure in which an aluminum atom is surrounded by six hydrogen atoms located at the corners, and each of the hydrogen atoms also occupies the corner of another octahedral structure.
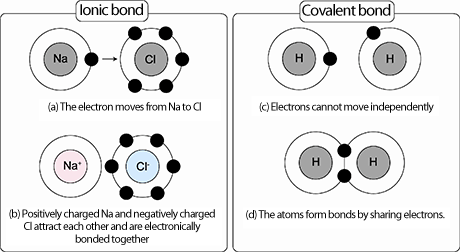
A typical ionic bond is observed in salt (NaCl). In NaCl, because the chlorine atom has an overwhelmingly higher affinity to electrons (•) than the sodium atom, an electron is transferred from the sodium atom to the chlorine atom (a), resulting in the chlorine atom being negatively charged and the sodium atom being positively charged (b). As a result, these atoms are bonded to each other through electrical attraction. This bonding state is called an ionic bond. On the other hand, a typical covalent bond is observed in hydrogen gas (H2). In H2 (c), because the two atoms become extremely stable if each atom gains one more electron, these atoms share electrons and are bonded together (d). This bonding state of atoms is called a covalent bond.
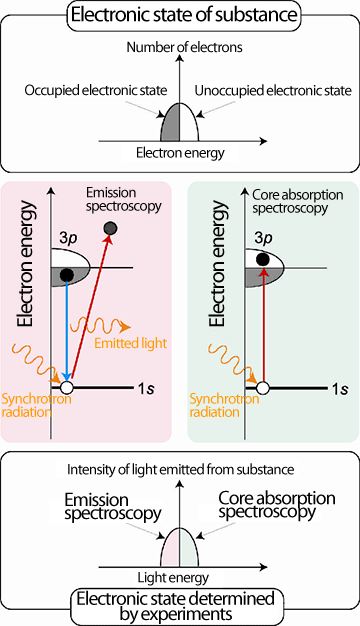
spectroscopy and core absorption spectroscopy
In absorption spectroscopy, the probability of Al 1s core electrons absorbing energy from synchrotron radiation and undergoing a transition to the unoccupied electronic state of Al 3p electrons is examined. In emission spectroscopy, the probability of Al 3p electrons in the occupied electronic state filling the space left by the Al 1s core electrons that have absorbed energy from the synchrotron radiation is examined. Combining these two experiments, the occupied and unoccupied electronic states of Al 3p electrons can be determined.
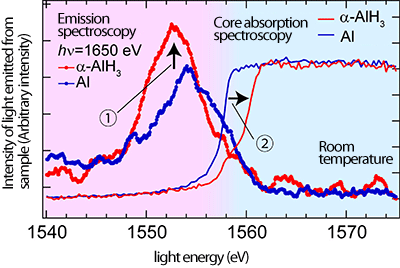
α-AlH3 (red) determined by emission spectroscopy
and core absorption spectroscopy
The horizontal axis in the results of emission spectroscopy (pink area) represents the energy of the light emitted from the sample upon irradiation with light of 1,650 eV. The horizontal axis in the results of core absorption spectroscopy (blue area) represents the energy of the light irradiated to the sample. The vertical axis represents the intensity of the light emitted from the sample in both experiments. The results of the two experiments are combined to determine the occupied and unoccupied electronic states of Al 3p electrons.
|
For more information, please contact: |
- Previous Article
- X-ray Crystallography of Oxygen-Tolerant Membrane-Bound [NiFe] Hydrogenase (Press Release)
- Current article
- Clarifying the Bonding State of Aluminum Storing a Significant Amount of Hydrogen Using Synchrotron Radiation (Press Release)