New Direction for Designing Catalysts for Automobile Exhaust Gas Purification Using Cuprates (Press Release)
- Release Date
- 01 Feb, 2012
- BL14B1 (JAEA Materials Science)
- BL14B2 (Engineering Science Research II)
- BL28B2 (White Beam X-ray Diffraction)
Osaka University
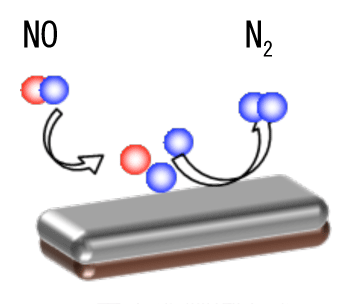
Scientists of the "New Development of Novel Self-Forming Nanoparticle Catalyst without Precious Metals"*3 research group have clarified the mechanism underlying the activity of a catalyst used to purify automobile exhaust gases*2 and obtained a new direction for designing novel catalysts on the basis of the findings. This research is part of the Elements Science and Technology Project*1 supported by the Ministry of Education‚ Culture‚ Sports‚ Science and Technology. The core organization in this project is Japan Atomic Energy Agency (Project Leader‚ Yasuo Nishihata)‚ and the other participating organizations are Osaka University (Chief Scientist‚ Hideaki Kasai)‚ Daihatsu Motor‚ Co.‚ Ltd. (Chief Scientist‚ Hirohisa Tanaka)‚ and Hokko Chemical Industry‚ Co.‚ Ltd. (Chief Scientist‚ Senshu Mitachi). A press conference was held to announce the findings on (Tuesday) 7 February 2012. Currently‚ precious metals such as rhodium (Rh)‚ palladium (Pd)‚ and platinum (Pt) are used as catalysts for the purification of automobile exhaust gases. The scientists of the research group theoretically analyzed the purification activity of catalysts containing transition metals*8 and performed demonstrative experiments using the SPring-8 beamlines BL14B1‚ BL14B2‚ and BL28B2‚ with the aim of designing novel catalysts that do not contain rare precious metals. They evaluated the interaction between nitrogen monoxide (NO)*4 and Rh‚ which is a precious metal exhibiting particularly high activity in the purification of NO contained in automobile exhaust gases‚ in addition to clarifying the differences between Rh and other precious metals and investigating the factors promoting the reduction*5 of NO. From the results obtained‚ they found that Rh has a dissociative adsorption*6 structure more stable than that of other precious metals‚ as well as a smaller activation barrier*7 of dissociative adsorption‚ which allows NO to be easily separated. The scientists at Osaka University theoretically analyzed the dissociative adsorption of NO on the surfaces of various transition metals‚ such as nickel (Ni)‚ cobalt (Co)‚ iron (Fe)‚ and copper (Cu)‚ and their oxides. The results revealed that the surfaces of Ni‚ Co‚ and Fe were easily oxidized in the presence of oxygen (O) and that O atoms adsorbing onto the surfaces disturbed the dissociative adsorption of NO. On the other hand‚ the surface of Cu more easily became exposed than that of the other transition metals‚ and the purification activity of Cu was inferior to that of Rh even though NO was reduced on the exposed Cu surface. Moreover‚ the reason for the less active dissociative adsorption of NO by Cu was found to be due to the electron state*9 of the Cu surface. The scientists examined the electron state and catalytic activity of copper(I) oxide (Cu2O)‚ a cuprate‚*10 to realize Cu with a similar electron state on its surface to that of Rh. They designed a structure that can be realized by oxidizing Cu then removing O atoms from the Cu surface and found that this structure promoted the dissociative adsorption of NO. From the above theoretical findings‚ a new direction for designing novel catalysts for the purification of NOx was obtained‚ which is expected to lead to the decreased use of precious metals. Significant advances in the practical application of new catalysts are also expected to be made on the basis of the findings. The results of this study were presented on 15 February at nano tech 2012‚*12 The 11th International Nanotechnology Exhibition and Conference‚ which is the world’s largest fair of state-of-the-art nanotechnology.*11 |
<<Figures>>
Controlled surface of cuprate
<<Glossary>>
*1 Elements Science and Technology Project
This is a project supported by the Ministry of Education‚ Culture‚ Sports‚ Science and Technology. Its aims are to develop substances and materials with advanced functions that do not require rare or toxic elements by studying the roles and characteristics of elements that constitute substances and materials and to determine their functions and properties through clarification of their underlying mechanisms.
*2 Automobile exhaust gas purification catalysts
Automobile exhaust gas purification catalysts are used for removing the toxic components of automobile exhaust gases by promoting redox reactions. The purification device is installed midway along an exhaust pipe.
*3 Precious metals
In general, precious metals refer to elements such as gold (Au)‚ silver (Ag)‚ Pt‚ Pd‚ Rh‚ iridium (Ir)‚ ruthenium (Ru)‚ and osmium (Os). Precious metals are rare and resistant to corrosion.
*4 Nitrogen monoxide (NO)
NO is an inorganic compound consisting of N and O and is also known as nitrogen oxide. NO is generated during the combustion of organic compounds and is immediately oxidized when exposed to oxygen to become nitrogen dioxide (NO2). NO is used as a raw material in the production of nitric acid and is a cause of photochemical smog and acid rain.
*5 Reduction
Reduction refers to chemical reactions in which a substance receives an electron or electrons or the formal oxidation number of an atom decreases. More concretely‚ the substance is deprived of O or combined with hydrogen (H).
*6 Dissociative adsorption
Dissociative adsorption is a type of chemical adsorption. Molecules are decomposed‚ and then the products of the decomposition adsorb onto the surface of a substance.
*7 Activation barrier
When a system of substances in the equilibrium state transits to another equilibrium state‚ it must pass through a state with a potential energy higher than those of the two equilibrium states‚ which is referred to as an energy barrier. The difference between the potential energy of the intermediate state and that of the initial equilibrium state is called the activation barrier.
*8 Transition metals
Transition metals refer to the elements in the third to twelfth columns of the periodic table.
*9 Electron state
The electron state is the state of electrons in an atom or molecule. There are many properties that indicate the electron state‚ including the electron charge density (electron charge distribution)‚ band structure (or electron level)‚ magnetic structure (or electron spin state)‚ Fermi surface‚ state density‚ and the state of bonding between atoms (relationship with the electron charge distribution).
*10 Cuprates
Cuprates refer to copper oxides. There are two types of cuprate with different compositions‚ copper(I) oxide (Cu2O) and copper(II) oxide (CuO).
*11 Nanotechnology
Nanotechnology‚ also called nanotech‚ can be used to freely control substances at the nanometer (nm) scale‚ i.e.‚ at the atomic or molecular level (1 nm = 10-9 m). New materials and devices are developed at this scale using nanotechnology.
*12 nano tech 2012
nano tech is the world's largest exhibition of nanotechnology‚ where cutting-edge technologies and products from various nanotech-applied fields are displayed‚ such as nanomaterial development‚ ultrafine processing‚ evaluation‚ and measurement. nano tech 2012 was held in tandem with the following seven concurrent exhibitions: InterAqua 2012‚ ASTEC 2012‚ METEC 2012‚ Convertech JAPAN 2012‚ neo functional material 2012‚ Printable Electronics 2012‚ and Eco Cell Battery Exposition 2012.
|
For more information‚ please contact: |
- Previous Article
- Clarifying the Hidden Magnetism of Gold (Au) (Press Release)
- Current article
- New Direction for Designing Catalysts for Automobile Exhaust Gas Purification Using Cuprates (Press Release)