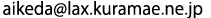Discovery of Tetravalent Cerium Binuclear Complexes Stable in Aqueous Solution (Press Release)
- Release Date
- 18 May, 2012
- BL11XU (JAEA Quantum Dynamics)
Japan Atomic Energy Agency
Helmholtz Association of German Research Centres, Dresden-Rossendorf
Synopsis
• The state of existence of tetravalent cerium in an aqueous solution was elucidated by means of a synchrotron radiation X-ray and quantum chemical calculations.
• Tetravalent cerium in an acidic aqueous solution was found to take the form of a binuclear complex, not the mononuclear complex commonly found in metallic ion complexes.
• The findings are expected to make a contribution for the advancement of application studies on aqueous Ce (IV) solutions, which directly relates to breakup reactions of water molecules in contact with a metallic catalyst, and therefore raises expectations as a method for clean hydrogen and oxygen generation.
|
The research group at Japan Atomic Energy Agency (JAEA, Director Atsuyuki Suzuki), in collaboration with the researchers at Helmholtz Association of German Research Centers in Dresden-Rossendorf (hereafter HZDR: Helmholtz-Zentrum Dresden-Rossendorf), conducted research on a soluble complex*1 of tetravalent cerium (IV), and elucidated its chemical structure in an aqueous solution. Cerium is known to be a unique element, among all the rare-earth elements,*2 that has both trivalent and tetravalent oxidation states that can exist stably in an aqueous solution. The aqueous reduction of cerium—Ce (IV) to Ce (III)—involves a very large reductive potential (approximately from 1.6 to 1.7V), making the Ce (IV) solution a powerful oxidative reagent. It serves as an indispensable oxidizing agent in a variety of applications in a range of areas, including organic synthesis. In recent years, aqueous Ce (IV) solutions, powerful oxidative agents, have been heavily used to activate metallic catalysts, especially in catalytic studies aiming at evolving hydrogen and oxygen from water molecules. Although aqueous Ce (IV) solutions have proved quite useful as chemical agents, little has been known about the chemical state of Ce (IV) in an aqueous solution, meaning that the reaction mechanisms involved in oxidation/reduction processes in an aqueous Ce (IV) solution have remained almost unresolved. As a representative Ce (IV) solution, the research group prepared Ce (IV) containing an aqueous perchloric solution (HClO4) through electrochemical means for X-ray absorption analysis. The X-ray absorption experiment*3 was carried out using one of the JAEA’s quantum dynamics beam lines (BL11XU) at the large-scale synchronous radiation facility SPring-8.*4 The analysis revealed that Ce (IV) exists in an aqueous solution as a peculiar binuclear complex, i.e. a bridged structure linked by way of the oxo*5 or hydroxyl group,*6 rather than as a mononuclear hydrated complex commonly formed by other metallic ions. The experimental results from X-ray absorption analysis were used in theoretical calculation using DFT (Density Functional Theory).*7 The combinational approach involving both experimental and theoretical methods elucidated, for the first time in the world, the detailed structure of the Ce (IV) binuclear complex. Dissolved Ce (IV) has long been considered to form mononuclear complexes, and the variety of oxidation reactions that take place in aqueous Ce (IV) solutions have been ascribed to the oxidative power of mononuclear Ce (IV) complexes. The outcomes of this research made it clear that Ce (IV) could form a stable binuclear complex in an aqueous solution, and it has been suggested that the linkage site via the oxo/hydroxyl group was chemically active. These findings suggest that the chemical activity of an aqueous Ce (IV) solution as an oxidant involves not only the transition from Ce (IV) to Ce (III) that accompanies single-electron reaction (a conventionally-assumed mechanism), but also the autonomous generation of oxygen from dissolved Ce-O binding, indicating the possibility that the oxidative catalyst reactions of water may stem from the activity of the oxo group incorporated in a binuclear complex. This constitutes basic knowledge for the understanding of reaction mechanisms of various chemical processes involving aqueous Ce (IV) solutions, typically the decomposition reaction of a water molecule. A deeper understanding of these phenomena is expected to play an important role for the development of the fuel-sourcing chemical reactions used in fuel cells, i.e. the precious metal catalyzed oxygen generating reactions that should endure repetitive use with little or no performance degradation. The results reported here are based on the collaborative study conducted by the following researchers at two research organizations: JAEA: Dr. Atsushi Ikeda (currently postdoctoral fellow at HZRD), Dr. Tsuyoshi Yaita (principal researcher), HZRD: Dr. Satoru Shimazu (research fellow), Dr. Christoph Henning (senior research fellow) and Professor Gert Bernhard. Part of the study, consisting of a series of experiments using synchrotron radiation X-ray spectroscopy, was conducted as a research proposal submitted for the use of the JAEA beam line (BL11XU) at SPring-8. The results of the research were issued in the online publication of “Dalton Transactions” (a publication from Royal Society of Chemistry, UK) on March 8, and the full paper will be published in its printed version (it will appear on the front cover of the issue). The research was also covered by the online publication of “Chemistry World” (an information publication from the Royal Society of Chemistry for general chemists) as a feature article. Publication: |
<<Figures>>
(2 mol/L-HClO4)obtained from synchrotron radiation X-ray absorption analysis of Ce’s K-shell,
and the plots of radial structural function simulations of Ce (IV)’s binuclear and trinuclear complexes
(“Dimer 1” to “Trimer”) as optimized by applying the density functional method (DFT).
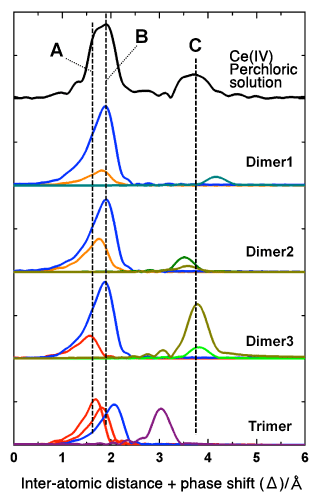
The black plot (top) represents the radial structure function of Ce (IV) in an aqueous perchloric solution. The peak “C” in the figure indicates that at least two Ce atoms are located very close to each other, evidencing that Ce (IV) forms multi-nuclear, rather than mononuclear, dissolved complexes. Comparison of the black plot with the theoretical plots of radial structure functions (optimized using DFT)—Dimer 1, Dimer 2, and Dimer 3 for binuclear complexes, and Trimer for a trinuclear complex—indicates that Dimer 3 (a binuclear complex with a bridged structure linked through oxo groups) gives the best fit with the black plot in terms of intensities and locations of peaks A to C.
optimized for the aqueous solution using the DFT method.
The designations of each structure (Dimer 1, Dimer 2, Dimer 3, and Trimer) in this figure correspond to the labels shown in Fig. 1. In this research, the information on these complex structures obtained from quantum chemical calculations was further used to simulate the radial structure function, leading to successful determination of three-dimensional complex structures. Data from X-ray absorption experiments alone would not have enabled such achievement.
<<Glossary>>
*1 Complex
A generic term describing a compound in which molecules or ions (called a ligand) form coordinated bonds to a metal ion. Complex ions, as the name implies, are positively or negatively charged compounds consisting of a metal ion and coordinated ligands. The coordination of negative ions to a metal ion (normally positively charged) can cancel the charge and make a neutral compound. The term “complex” normally includes both charged and neutral coordinated compounds.
*2 Rare-earth elements, lanthanoid elements
The 15 elements in the periodic table from lantern (57La) to lutetium (71Lu) inclusive are called “lanthanoid elements.” If scandium (21Sc) and yttrium (39Y) are added to the lanthanoid elements, these 17 elements are collectively called “rare-earth elements.” Cerium, the target of this study, is one of the rare-earth elements and, as a powerful oxidative agent, has widespread applications in a variety of areas in chemical industries, typically for organic synthesis.
*3 X-ray absorption spectroscopy
X-ray absorption spectroscopy is a widely used method to analyze the chemical state (oxidation number, complex structure, etc.) of constituting elements of matter. The sample is irradiated by an X-ray, typically generated in synchrotron radiation facility, and the amount of the beam taken up by it (absorption coefficient) or the strength of the fluorescent X-ray emitted from it is measured. As the method is applicable, in principle at least, to all elements and a wide variety of sample geometries, it has found very wide utilization in many areas, including physics, chemistry, and biology, in basic research as well as in applied studies.
*4 SPring-8
A RIKEN facility located in Harima Science Garden City (Hyogo prefecture) is capable of producing the world's highest intensity synchronous radiation. The management and promotion of utilization of this facility are undertaken by JASRI. The name “SPring-8” comes from “Super Photon ring-8GeV.” An electron flying at nearly the speed of light, if deflected from its original trajectory through the effect exerted by a magnet, emits an electromagnetic wave in a direction tangential to its trajectory, which is called radiation light (or synchrotron radiation). At present, there are three “3rd Generation” large scale synchronous radiation facilities in the world: SPring-8 (Japan), APS (USA) and ESRF (France). The acceleration energy available at SPring-8 (8 billion electron volts) enables the provision of an extremely wide spectrum of radiation light: from far infrared to visible, vacuum ultraviolet, and soft X-ray up to hard X-ray. SPring-8 provides a theater for collaborative works involving researchers inside and outside Japan, and the research conducted at this facility cover such diverse areas as material science, geoscience, life science, environmental science, and various applications in industrial sectors.
*5 Oxo groups
A generic term describing the oxygen-based functional groups characterized by their bonding state to a metallic ion as “=O2–.” In addition to the oxo-groups found in the cross-linked structures covered by this research, they also occur in such oxo-anions as molybdate ion (MoO42–) and dichromate ion (Cr2O72–).
*6 Hydroxyl groups
A generic term describing the type of functional groups that bind to a metallic and other ion in the form characterized by “-OH–.” The hydrolysis of water molecules in an aqueous solution generates hydroxide ions (OH–), making them one of the most common ligands observed in aqueous systems.
*7 Density-functional method
A theoretical method to calculate the electronic state and chemical structure of atoms and molecules using the methodology called DFT (Density Functional Theory), which is a branch of quantum mechanical theory applicable to the study of electronic states of many-electron systems including atoms and molecules. In this research, the method was used to estimate the chemical structure of binuclear and trinuclear complexes that may be formed by Ce (IV) in an aqueous solution.
|
For more information, please contact:
Dr. Atsushi Ikeda (Helmholtz-Zentrum Dresden-Rossendorf) |
- Previous Article
- Elucidation of Atomic-level Mechanisms in Which a Pair of Hydrogen-ion Permeable Proteins Function (Press Release)
- Current article
- Discovery of Tetravalent Cerium Binuclear Complexes Stable in Aqueous Solution (Press Release)
 /
/