Direct atomic-scale observation of mechanism of mutual influence between solute and solvent - Tracking structural changes in solute and solvation in photochemical reactions on 10-femtosecond timescale -(Press Release)
- Release Date
- 14 Feb, 2023
- SACLA BL3
February 14, 2023
Japan Synchrotron Radiation Research Institute (JASRI)
RIKEN
High Energy Accelerator Research Organization (KEK)
A collaborative research group led by Tetsuo Katayama (research scientist) of the XFEL Utilization Division, Japan Synchrotron Radiation Research Institute (JASRI), has succeeded in clarifying the atomic-scale mechanisms of photochemical processes in solution, where the solute molecules absorbing light and the surrounding solvent molecules influence each other. In solution, the reactants (solute molecules) are surrounded by solvent molecules. This leads to a diversity of the speed of chemical reactions, the lifetime of intermediates(*1), and the type and yield of products depending on the solvent (solvation effects)(*2). However, the effect of solvent molecules promoting or suppressing the structural changes of solute molecules and the chemical reactions have not been directly observed. This research group accurately tracked the structural changes in the metal complexes(*3)(solute molecules) absorbing light and the acetonitrile molecules (solvent molecules) using BL3 of the X-ray free-electron laser (XFEL) facility, SACLA(*4). As a result, they found that the motions of solute molecules trigger the rearrangement of surrounding solvent molecules and that such rearrangement, in turn, drives the motions of solute molecules. They observed the mutual influence between solute and solvent molecules on an atomic scale. In this study, a movie for observing the structural changes in solute and solvent molecules was obtained, contributing to the understanding of the mechanisms behind solvation effects in photochemical reactions. Also, this study is a sequel to the group's study published in the scientific journal Nature Communications in 2019. The achievements of this study were published in the online edition of the scientific journal Chemical Science. Publication information |
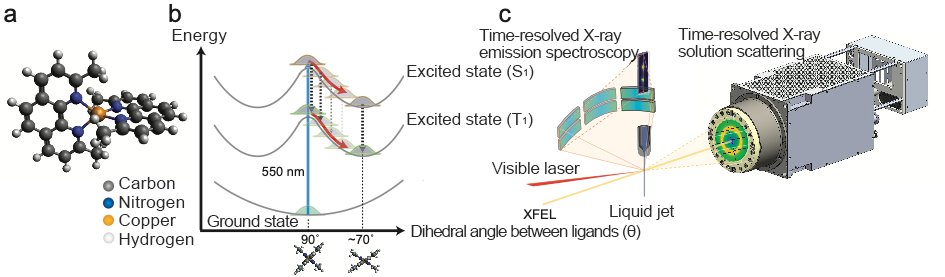
Fig. 1:(a) Structure of Cu(I)-phenanthroline complex. Two phenanthroline ligands are orthogonally attached to the central Cu atom. (b) Schematics of the reaction pathway and energy (potential energy surfaces) of Cu(I)-phenanthroline complex. The initial tetrahedral structure becomes unstable upon absorbing visible light and the complex flattens (c) Experimental setup for time-resolved X-ray solution scattering and X-ray emission spectroscopy measurements.
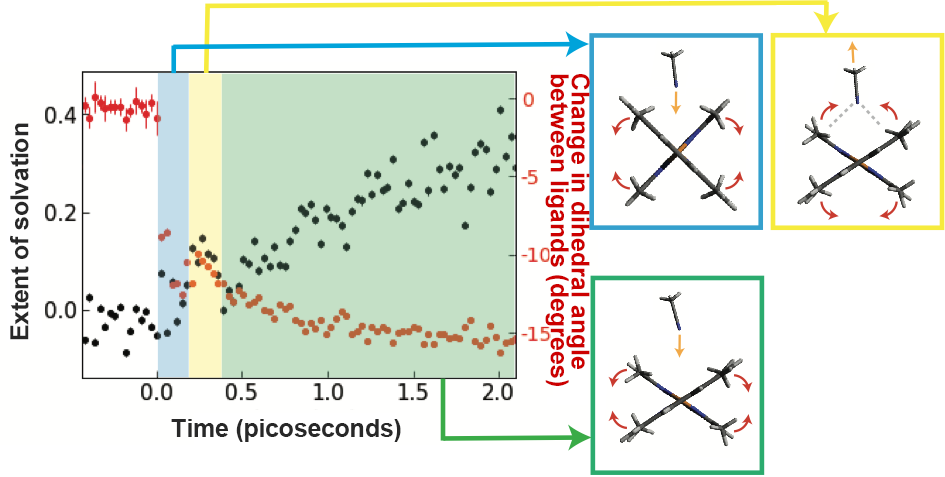
Fig. 2:Extent of solvation; Time (picoseconds); Change in dihedral angle between ligands (degrees)
[Glossary]
※1. Intermediates
Substances temporarily formed during the course of chemical reactions from the initial reactants to the final products. The more unstable the reaction intermediates, the shorter their lifetime.
※2. Solvation effects
Effects of solvent on the responsiveness (the stabilities of reactants, reaction intermediates, and products) in chemical reactions in solution. The solvation effects are governed by various intermolecular interactions between solute and solvent molecules.
※3. The metal complexes
A compound that has a structure in which a non-metal molecule or ion is bonded to a metal ion.
※4. X-ray free-electron laser facility, SACLA
Japan’s first XFEL constructed jointly by RIKEN and JASRI. SACLA is one of the five national core technologies in Japan’s Third Science and Technology Basic Plan. The construction and preparation of SACLA were launched in FY2006 as a five-year project and completed in March 2011. The name SACLA is short for SPring-8 Angstrom Compact free electron LAser. In June 2011, the first oscillation of the X-ray laser was achieved. Since March 2012, SACLA has been open to public users. SACLA is capable of generating lasers with the world’s shortest wavelength of ≤0.1 nm.
《Contaact》 |
- Current article
- Direct atomic-scale observation of mechanism of mutual influence between solute and solvent - Tracking structural changes in solute and solvation in photochemical reactions on 10-femtosecond timescale -(Press Release)
 spring8.or.jp
spring8.or.jp