Topic 18: Controlling Electronic States through the Spin–Orbit Interaction of Electrons
Strange States of the Outermost Electrons - Key to Developing New Technologies
Although electronic states determine the properties of a material, the actual states are unexpectedly difficult to reveal, especially the states of the outermost electrons (electrons in the outermost shell) of the constituent atoms in a material. However, these states are necessary to elucidate the properties of high-temperature superconductors and ferromagnetic materials as well as those of transition metal oxides exhibiting nonconductive properties. Unfortunately, relatively little information has been obtained about the electronic states due to limitations in the examination methods, but this has changed due to the work of Dr. Hidenori Takagi (Professor, The University of Tokyo, Japan), Dr. Takahisa Arima (Professor, Tohoku University, Japan), and colleagues.They used the highly brilliant synchrotron X-rays at SPring-8 to examine the states of the outermost electrons, and discovered materials capable of pioneering a field, spintronics.
Strong Correlation between the Direction of Electron Spin and Orbital Motion
An oversimplified explanation of the motion of electrons can be described as follows. Electrons spin around their own axis while rotating around nucleus, which is at the center of an atom. The former motion is called spin and the latter is called orbital motion. Individual electrons are like small magnets. The electron spin and orbital motion in an isolated atom interact. In contrast, the outermost electrons are affected by neighboring atoms in a solid where atoms are densely populated. Thus, opposite directions (clockwise (right-handed) and counter-clockwise (left-handed)) of electronic orbital motions around a nucleus occur with almost the same probability, and thereby cancel each other. This causes the overall electronic orbital motion to nearly disappear. In 1986, high-temperature superconductors were discovered. Since then oxides of 3d transition metals (titanium, manganese, iron, and copper) have been attracting attention as high-temperature superconductive materials. Because the outermost electrons terminate orbital motions, they assume a borderline state where some can but others cannot move to neighboring atoms. The functions of these outermost electrons are thought to be responsible for the superconductivities and ultra-giant magnetoresistance in the oxides of 3d transition elements. In the Periodic Table, 3d transition elements lie above 4d and 5d transition elements. The ease of moving the outermost electrons to neighboring atoms has been speculated to increase as the atomic number increases (4d, 5d, etc.). Thus, oxides with larger atomic numbers would lose superconductivity and the ultra-giant magnetoresistance observed in 3d transition elements. However, recent studies have revealed new findings contrary to this hypothesis. For example, when iridium (Ir) in Sr2IrO4 (an insulator) is replaced with rhodium (Rh) or cobalt (Co), where Co, Rh, and Ir form a column in that order in the Periodic Table, both Sr2RhO4 and Sr2CoO4 become metals (conductors) although they have the same crystal structure as Sr2IrO4. It has been a mystery why Sr2IrO4 (iridium oxide), the oxide of a 5d transition element whose outermost electrons are supposed to move easily, is an insulator.
“We tried to explain this mystery based on the concept of resurgence of orbital rotation. The interactions between the direction of electron spin and that of orbital motion are stronger for heavier elements with larger atomic numbers. In iridium oxide (Sr2IrO4) and similar oxides, the effect of spin-orbit interactions is sufficient to surpass the effects from neighboring atoms. Thus, the possibility of recovering the electronic orbital motions is high. If electron transfer to neighboring atoms is suppressed due to this effect, then conductivity would be lost. This could explain why Sr2IrO4 is an insulator. However, it has been extremely difficult with existing techniques using magnetism to demonstrate whether the orbital motions of the outermost electrons are recovered because the magnetism of Sr2IrO4 is extremely weak,” describes Dr. Takagi.
Spin and Orbital Motion of Outermost Electrons are Strangely Correlated
To examine the orbital motions of electrons that influence the conductivity and magnetism of Sr2IrO4, Dr. Takagi’s research group utilized synchrotron radiation X-ray diffraction at SPring-8. First, they determined the spin arrangement (magnetic structures of electrons) of this nonconductive oxide. Although neutron beams are commonly used to determine spin arrangements, iridium atoms absorb neutrons, rendering this method ineffectively. Moreover, determining spin arrangements solely using X-ray diffraction is extremely difficult and has yet to be successful.
Thus, Dr. Takagi and colleagues conducted experiments using X-rays at SPring-8. They used specific wavelengths where the X-ray diffraction signals from the electron spins of the iridium atoms are amplified to successfully determine the spin arrangements. X-ray diffraction requires a smaller sample size compared to techniques using neutron beams. Hence, it is anticipated that SPring-8 will play a wider role in determining spin arrangements in the future.
Strong spin-orbit interactions occur in 5d transition elements. Thus, the electron arrangement of iridium with right-handed orbital motion and that with left-handed exhibit the same arrangement pattern as the spin arrangement. Therefore, their research group successfully demonstrated that when spin and orbital motion have the same period, X-ray waves diffracted by electron spin and those diffracted by electronic orbital motion interfere with each other depending on the wavelength of X-ray (Fig. 1). Thus, the intensity of the diffracted waves depends on whether the wavelengths for spin and orbital motion have the same period (Fig. 1). Experiments conducted using extremely strong synchrotron radiation X-rays with adjustable energy (wavelength) at SPring-8 revealed that a significantly enhanced resonance (amplification of X-ray scattering intensity) is observed at the longer wavelength (L3 edge), but the resonance is negligible at the shorter wavelength (L2 edge) (Fig. 2).
Their experimental results reflect the interference effects of electronic orbital motion waves in resonance. Further detailed theoretical analyses revealed the probability of observing an outermost iridium electron orbiting in the same direction as its spin is about two-thirds, whereas the probability of the opposite (and the orbital motion ceases) is about one-third (Fig. 3). This strange spin-orbit correlation, which is not observed in isolated atoms, is unique to the outermost electrons of Sr2IrO4. Thus, the transfer of electrons to neighboring atoms is suppressed, making Sr2IrO4 an insulator. In the past materials science research on physical properties mainly targeted oxides of elements with small atomic numbers, such as oxides of manganese, iron, and copper (3d transition metal oxides). Recently, research on the physical properties and functions of 5d transition metal oxides has been receiving increased attention. The properties and functions of 5d transition metal oxides have completely different orders of magnitude due to the much stronger electron spin-orbit interactions than those of 3d transition metal oxides.
“Directly observing spin-orbit interactions using resonance phenomena is very significant. Our success will lead to increased collaborations between theoretical research on the physical properties of materials and advanced measurement technologies,” explains Dr. Takagi. If spintronic circuit elements, which control spin but not electric charge, are achieved, then the development of revolutionary energy-saving devices will become a reality. The development of electron spin control, which is still in the trial stage, requires a thorough understanding of spin-orbit interactions. The stronger the spin-orbit correlation, the easier it is to control the electronic/optical of spin information; therefore, electron spin control in 5d transition metal oxides may be realized if breakthrough control techniques are developed.
Their research achievement was published in Science (March 5, 2009), and in 2010 Dr. Takagi received the Prize for Science and Technology (Research Category), the Commendation for Science and Technology by the Minister of Education, Culture, Sports, Science and Technology of Japan, for his achievements and contributions.
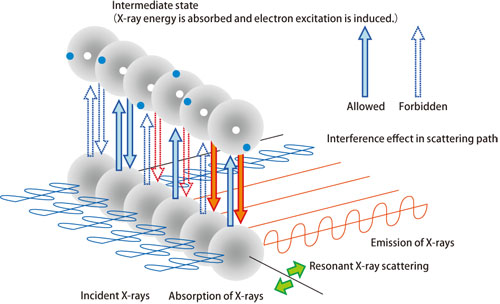
Diffraction occurs due to the superposition of scattering X-rays from individual atoms. When the energy of the incident X-rays equals the excitation energy of an electron in an atom, the resonance between the incident and absorbed/emitted X-rays amplifies X-ray scattering. This results in an increased diffraction intensity. If there is a phase difference (time lag) between these two types of X-rays, then the resonance may be canceled due to interference.
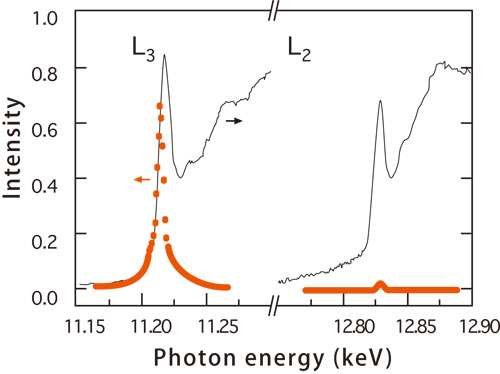
Rising edge of X-ray absorption (red circles) corresponds to the electron transition where the resonance in diffraction intensity (black solid lines) is maximized. Substantial increase occurs at the L3 edge, while the increase at the L2 edge is negligible. Relationship between energy and wavelength is roughly expressed as wavelength (nm = 10-9 m) × photon energy (keV = 103 eV) = 1.24 where the energy of the incident X-rays is expressed in terms of photon energy (keV) on the horizontal axis.
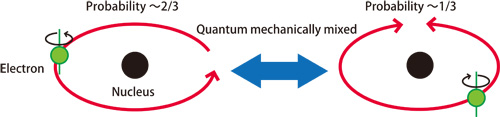
Probability of observing an electron orbiting in the opposite direction as the direction of its spin is about one-third. Orbital motion ceases when the electron orbits opposite to the spin direction. In the right figure, spin and orbital motion have opposite directions.
Reference
1. B. J. Kim, H. Ohsumi, T. Komesu, S. Sakai, T. Morita, H. Takagi and T. Arima; Science, 323, 1329 (2009)