Topic 17: Hard X-ray Photoelectron Spectroscopy (HAXPES)
Unraveling Mystery that Puzzled Dr. Mott, a Nobel Laureate
Insulators are materials that resist the flow of electric current. The most prevalent are band insulators, which cannot conduct electric current due to intrinsically lacking free-moving electrons. However, for unknown reasons some materials such as nickel oxide, which possess free electrons and should be conductors according to common knowledge in physics, exhibit the properties of insulators. Dr. Nevill F. Mott, who received the Nobel Prize in Physics in 1977, was fascinated by this mystery and tried to resolve it. This is why insulators such as nickel oxide are called Mott insulators. However, this mystery was only solved very recently by research conducted at SPing-8.
Why is Band Theory Not Applicable?
The behavior of the numerous electrons moving around in a solid characterizes various properties of the solid, including the electric properties. The energies of these electrons range from low to high. In a solid, electrons occupy distinct energy levels, forming a band structure. The aggregation of these electrons is called an “electron sea” or a “Fermi sea” (named after physicist Enrico Fermi), where all the bands below the uppermost level (Fermi energy) are filled with electrons.
In a Fermi sea, lower energy electrons stay near the bottom while higher energy electrons remain near the surface. Analogous to seawater where the normal sea moves more rapidly near the surface and more slowly near the bottom, a Fermi sea filled with electrons behaves similarly. Because the properties of each solid are thought to be characterized by a few electrons near the surface of the Fermi sea, a thorough understanding of the characteristics and states of such electrons is necessary to explore the properties of a solid.
Band theory, a basic theory of solid-state physics established around 1930, is used to describe and understand the states of various electrons in solid crystals. It describes the electronic states of materials with repeating regular structures of the same atomic arrangements based on quantum mechanics, and has contributed to the elucidation of physical phenomena of materials.
However, a strange problem arose. According to band theory nickel oxide should be a metal, but it behaves as an insulator and blocks electric current. Dr. Neville F. Mott, a leading authority in physics, and his colleagues wrestled with this mystery. They hypothesized that electrons near the surface of the Fermi sea of nickel oxide are in an overly dense state and cannot move freely (Fig. 1: a dark blue parts). Their idea is analogous to a very crowded main street where people in crowd cannot move individually.
In the first half of the 1980s, the situation drastically changed, and a completely different explanation for this mystery was proposed. Research indicated that nickel oxide is an insulator because electrons near the surface of the Fermi sea are oxygen electrons (Fig. 1: light blue parts) while nickel electrons exist in a deeper location far from the surface. However, this hypothesis did not resolve all the issues.
“A problem with the theory proposed in the 1980s (Fig. 2: red dashed line) is that it cannot reproduce the experimental peak position of the energy spectrum of the photoelectrons emitted from nickel oxide obtained experimentally using core-level hard X-ray photoelectron spectroscopy (Fig. 2: blue solid line). Although two peaks are experimentally observed, the theory predicts a single peak (peak positions of A and C do not coincide, and there is only one peak at C in Fig. 2). Moreover, the valence band soft X-ray photoelectron spectroscopy shows that the theory from the 1980s (Fig. 3 red dashed line) does not well reproduce the experimentally obtained energy dependency of the photoelectron spectra (Fig. 3 orange line). Thus, the theory is incomplete,” explains Dr. Shik Shin1) (Team Leader, the Excitation Order Research Team, Quantum Order Research Group, RIKEN SPring-8 Center, Japan).
1) Also Professor at the Institute for Solid State Physics, The University of Tokyo.
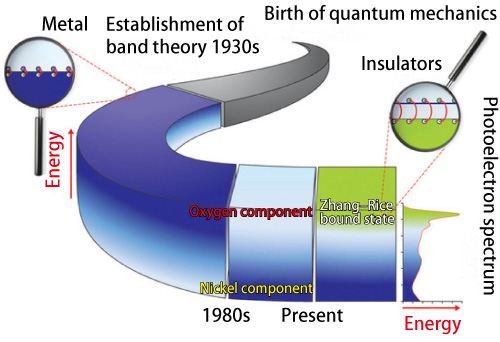
Many researchers have tried to decipher the mystery of nickel oxide, which is concurrently a metal and an insulator. Originally, nickel oxide was speculated to be an insulator because the electrons near the surface (dark blue) of the Fermi sea are highly condensed and cannot move freely. However in the 1980s, the understanding of the non-conductivity of nickel oxide drastically changed, and it was hypothesized the electrons near the surface are oxygen electrons (light blue). This theory had been commonly accepted until the Zhang-Rice bound state (green) was proposed to explain the mechanisms of copper-oxide high-temperature superconductors in 1988.
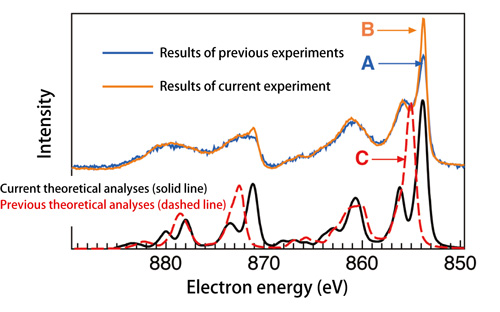
Peak C, which is a single peak in the previous analyses (red dashed line), is divided into two peaks in this current analyses (black solid line). Current analyses agree with the experimental data. Height of Peak A (blue) from the previous analyses increases to Peak B (orange) in this study, indicating that current theoretical analyses can nearly reproduce the experimental data in terms of intensities.
Hard X-rays at SPring-8 are Powerful Enough to Resolve the Mystery of Mott Insulators
Dr. Tetsuya Ishikawa (Director, RIKEN SPring-8 Center), Dr. Shin, Dr. Munetaka Taguchi (Research Scientist, RIKEN SPring-8 Center), and colleagues examined the properties of electrons near the surface of the Fermi sea of nickel oxide using core-level hard X-ray photoelectron spectroscopy and valence band soft X-ray photoelectron spectroscopy. Both techniques examine the properties of photoelectrons, which are electrons emitted from a material by X-ray irradiation. The former determines the properties of electrons near the surface of the Fermi sea by observing physical processes where electrons near the sea surface compensate for the “holes” created when individual electrons are extracted from the sea bottom upon irradiation with hard X-rays (high-energy X-rays with energy of 3-100 keV). In contrast, the latter examines the properties of electrons by directly extracting them from the sea surface using soft X-rays (X-rays with energy of 100-3,000 eV).
RIKEN Coherent X-ray Optics Beamline (BL29XU) enabled their research team to utilize core-level hard X-ray photoelectron spectroscopy. Hard X-rays allow researchers to examine the properties of electrons in a solid. Prior to the development of hard X-rays, existing core-level photoelectron spectroscopy could not probe the inside of solids because the low-energy X-rays could only penetrate near the surface of a solid. However, their research revealed nickel oxide possesses a unique electronic state, the Zhang-Rice bound state. The Zhang-Rice bound state is created through complicated interactions between nickel and oxygen in nickel oxide. This concept was theoretically driven by Dr. Fu-Chun Zhang2) and Dr. Thomas M. Rice (both at the Swiss Federal Institute of Technology) in 1988 to explain the mechanisms of copper-oxide high-temperature superconductors.
Dr. Ishikawa and colleagues demonstrated that the energy components induced by electrons under such a unique electronic state increase significantly to raise the peak intensity (Fig. 2: Peak B). Additionally, they examined the energy-dependent electronic states near the Fermi sea and the energy peak properties using a valence-band soft X-ray photoelectron spectroscopic system at the RIKEN Coherent Soft X-ray Spectroscopy Beamline (BL17SU). They found that the patterns in the energy spectra of photoelectrons obtained from current theoretical predictions (Fig. 3: solid black lines), which consider the electron components induced from the Zhang-Rice bound state, agree well with those obtained from the experimental data (Fig. 3: solid orange lines).
Dr. Shin, Dr. Taguchi, and colleagues have performed theoretical analyses that consistently explain these two different experimental results. They revealed that electrons under the Zhang-Rice bound state (Fig. 1: green part) are responsible for the conduction mechanism of nickel oxide. Additionally, oxygen electrons, which were thought to be responsible for the conductivity, exist in an area deeper than the surface, while nickel electrons exist in an even deeper area. Furthermore, this unique Zhang-Rice bound state of electrons, which also plays an important role in copper-oxide high-temperature superconductors, has been suggested to be responsible for charge-transfer type insulators in general.
“The precise understanding of the electronic states of materials is of top priority in developing innovative devices. Thus, this discovery that the non-conductivity of nickel oxide is induced by complex interactions between nickel and oxygen may be an important guide for employing various transition metal oxides as raw, thermoelectric materials in next generation electronics,” explains Dr. Shin. Their research achievements were published in Physical Review Letters (May 2008).
2) Currently Professor at The University of Hong Kong, China.
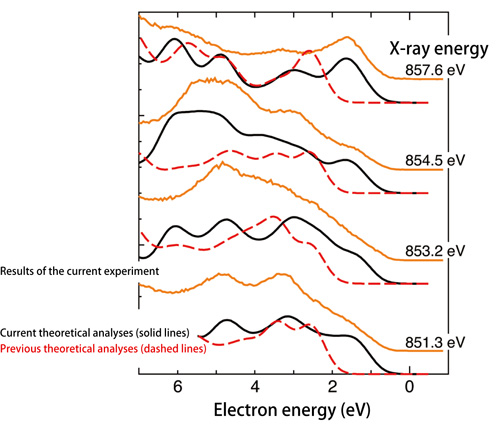
Electrons near the surface of the Fermi sea are directly observed using soft X-rays, which have energies less than hard X-rays. Soft X-rays with various energies were used to examine the energy dependency of photoelectron spectra. In contrast to previous theoretical analyses, current theoretical analyses, in which the components of the Zhang-Rice bound state are taken into account, nearly reproduce the energy-dependent experimental spectra in the low energy region (0-4 eV).
Reference
1. M. Taguchi, M. Matsunami, Y. Ishida, R. Eguchi, A. Chainani, Y. Takata, M. Yabashi, K. Tamasaku, Y. Nishino, T. Ishikawa, Y. Senba, H. Ohashi, and S. Shin; Phys. Rev. Lett., 100, 206401 (2008)