Structure determination of "selenomethionyl" rat heme oxygenase-1 by MAD experiment
Inquiry number
SOL-0000001092
Beamline
BL41XU (Macromolecular Crystallography I)
Scientific keywords
| A. Sample category | biology, medicine |
|---|---|
| B. Sample category (detail) | biomolecule, crystal, protein, pharmaceuticals |
| C. Technique | X-ray diffraction |
| D. Technique (detail) | single crystal, MAD |
| E. Particular condition | low-T (~ liquid N2) |
| F. Photon energy | X-ray (4-40 keV) |
| G. Target information | chemical state, chemical bonding, structure analysis, function and structure |
Industrial keywords
| level 1---Application area | Pharmaceuticals |
|---|---|
| level 2---Target | drug design, process analytical technology (PAT) |
| level 3---Target (detail) | protein, drug |
| level 4---Obtainable information | crystal structure, local structure |
| level 5---Technique | diffraction, X-ray diffraction |
Classification
M10.10 single crystal diffraction
Body text
Multiwavelength anomalous diffraction (MAD) method is the powerful method to obtain the phase information from one sample crystal composed of molecule with heavy atoms.
Most protein is composed by light atoms, such as carbon, oxygen, nitrogen and so on. In multiwavelength anomalous diffraction (MAD) experiment, therefore, it is necessary to bind some heavy atom to protein artificially. When the purpose protein is expressed as a selenomethionyl protein, it has some selen as heavy atoms intrinsically. It is not necessary to make heavy atom derivatives by using this selenomethionyl protein, and expected highly improvement of throughput.
In this case, structure of selenomethionyl heme oxygenase-1 from rat was determined by MAD experiment using Se.
This protein has eight methionines. The protein replaced all methionine to selenmethionine did not crystallized in reproducible. Four methionines were replaced to other amino acid by genomic engineering technique, and reminder were replaced to selenomethionine.
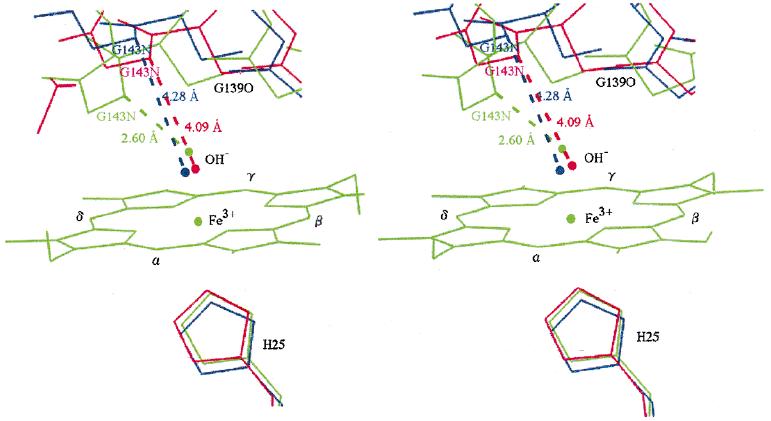
In this result, it could be discussed the differences of activities to substrates between this enzyme from rat and human (Fig. 1).
Figure 1. Stereo-view of heme oxygenase-I
(green: from rat, blue: crossed form from human, red: open form from human)
[ M. Sugishima, Y. Omata, Y. Kakuta, H. Sakamoto, M. Noguchi and K. Fukuyama, FEBS Letters 471, 61-66 (2000), Fig. 4,
©2000 Federation of European Biochemical Societies ]
Source of the figure
Original paper/Journal article
Journal title
M. Sugishima, et al., FEBS Lett. 471(1), 61-66, Apr. 7 (2000)
Figure No.
4
Technique
Multiwavelength anomalous dispersion (MAD) method is that the X-ray diffraction data are collected at near several energies of the absorption edge of heavy atom binding to molecule consisting crystal. By comparing and analyzing those diffraction data, the phase information is decided, and three-dimensional structure is able to determined.
Most protein is composed by light atoms, such as carbon, oxygen, nitrogen and so on. In multiwavelength anomalous dispersion (MAD) experiment, therefore, it is necessary to bind some heavy atom to protein artificially. It costs large time and labor to search species and conditions of heavy atoms bound to the protein.
Methionine is one of the amino acid composing the protein, and selenomethionine is synthesized amino acid that replaced sulfur atom of methionine to selen. When the methione auxotrophic E. Coli is grown in the medium containing selenomethionine instead of methione, all protein expressed by that E. coli are synthesized using selenomethione. This "selenomethionyl" protein has the selen as heavy atoms intrinsically. By using this "selenomethionyl" protein as a sample for MAD experiment, it is not necessary to make heavy atom derivatives, and expected highly improvement of throughput.
Source of the figure
No figure
Required time for experimental setup
0 hour(s)
Instruments
| Instrument | Purpose | Performance |
|---|---|---|
| Protein Crystal Diffractometer | To record diffraction data | |
| Peltier-cooled Si-PIN photodiode | To measure X-ray absorption spectrum |
References
| Document name |
|---|
| M. Sugishima, et al., FEBS Lett. 471(1), 61-66, Apr. 7 (2000) |
Related experimental techniques
Questionnaire
This solution is an application of a main instrument of the beamline.
Similar experiments account for more than 30% of the beamline's subject.
Ease of measurement
Middle
Ease of analysis
Middle
How many shifts were needed for taking whole data in the figure?
Two-three shifts