Determination of Steric Structure of Catalytic Protein for Nitrile Synthesis – Contribution to development of environmentally friendly nitrile production method using aldoxime dehydratase - (Press Release)
- Release Date
- 09 Nov, 2009
- BL41XU (Structural Biology I)
- BL44B2 (RIKEN Materials Science)
National Institutes of Natural Sciences (NINS) |
Key research achievements
• Contribution to establishment of nitrile synthesis method using biotechnology
• Successful preparation of an active enzyme-substrate complex and its structural analysis using SPring-8 synchrotron radiation
• Clarification of mechanism of conversion from aldoxime compounds to nitrile compounds
|
The National Institutes of Natural Sciences (NINS) (Yoshiro Shimura, President), RIKEN (Ryoji Noyori, President), and Toyama Prefectural University (Masato Tanaka, President) have determined the steric structure of aldoxime dehydratase (Oxd),*2 which catalyzes the synthesis of nitrile compounds*1 by a dehydration reaction, for the first time in the world. This was achieved by a joint research group consisting of Shigetoshi Aono, a professor at Okazaki Institute for Integrative Bioscience, NINS; Hitomi Sawai, a research fellow of the Japan Society for the Promotion of Science; Yoshitsugu Shiro, a chief scientist, and Hiroshi Sugimoto, a senior research scientist, at the Biomedical Science Laboratory of RIKEN SPring-8 Center; and Yasuhisa Asano and Yasuo Kato, professors at the Biotechnology Research Center, Faculty of Engineering, Toyama Prefectural University. Nitrile compounds are basic ingredients of various compounds and products such as organic solvents, acrylic fibers, nylon and herbicides. However, harsh reaction conditions, namely, acidity and high temperature, are required for the synthesis of nitrile compounds from their precursors, aldoxime compounds, by the conventional chemical synthetic procedure. On the other hand, it has recently been revealed that, in the natural world, nitrile compounds are efficiently synthesized under mild conditions because an enzyme named Oxd found in soil microorganisms acts as a catalyst. This research group has determined the steric structure of Oxd at the atomic level by X-ray crystal structural analysis*3 using the Structural Biology I Beamline (BL41XU) and the RIKEN Structural Biology II Beamline (BL44B2) (renamed RIKEN Materials Science Beamline in May 2009) at SPring-8, and discovered a new type of folded structure. Also, they succeeded in observing the intermediate reaction stages of Oxd by creating an active enzyme through a constant dose of X-ray radiation to Oxd crystals at an ultralow temperature. The clarification of the reaction mechanism and the substrate binding pattern of Oxd in this study is expected to contribute to the production technique of nitrile compounds using biotechnology at a low environmental cost. These research achievements were published in the American biochemical journal, Journal of Biological Chemistry, on 13 November 2009, and the paper was chosen as Paper of the Week. Publication: |
<Figure>
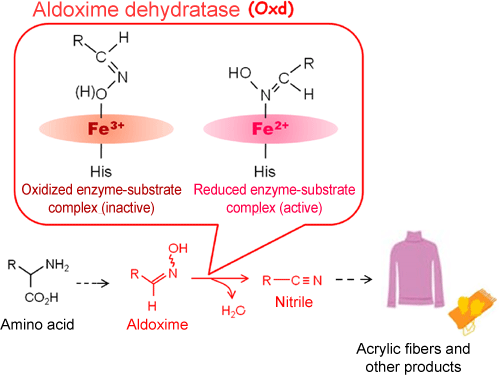
Fig. 1 Aldoxime-nitrile metabolic pathway in soil bacteria and activity control mechanism unique to Oxd
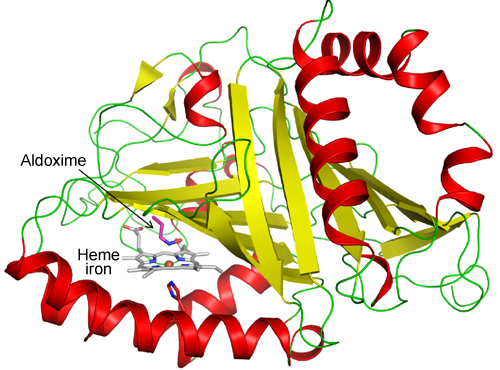
Fig. 2 Structure of entire Oxd enzyme
β strands (yellow) form a β barrel with a new structure. Heme iron is located between the β barrel and the helix (red).
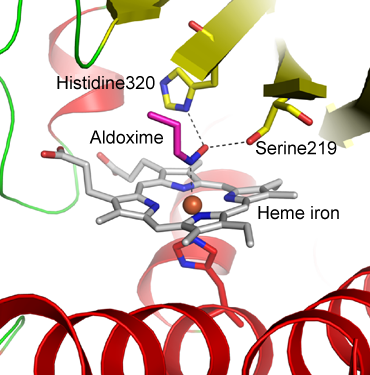
Fig. 3 Substrate-binding site of active enzyme-substrate complex
Substrate aldoxime not only binds to heme iron but is also fixed by the hydrogen bonds of serine and histidine, which are the 219th and 320th residues, respectively, from the amino terminal.
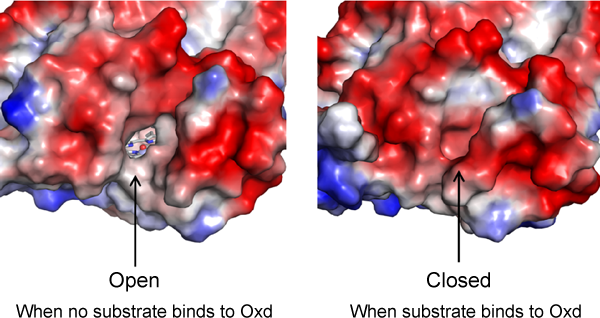
Fig. 4 Comparison of surfaces of substrate-free (left) and substrate binding (right) molecules
When no substrate binds to Oxd, there is a hole from the molecular surface to the substrate-binding site (space around heme iron); once a substrate binds to Oxd, the hole is closed. The red and blue parts of the molecular surface are negatively and positively charged, respectively.
<Glossary>
*1 Nitrile compounds
The generic term for organic compounds having the structure R-C≡N.
*2 Aldoxime dehydratase
The enzyme that catalyzes the reaction producing a nitrile compound and a water molecule from an aldoxime compound, which has the structure R-C=N-OH.
*3 X-ray crystal structural analysis
By irradiating a short-wavelength X-ray to a crystal, which has a regularly ordered structure, and analyzing the intensity of the diffracted X-ray in detail, the molecular structure of the crystal can be determined. The steric structure of many biomolecules has been thus determined.
|
For more information, please contact: |
- Current article
- Determination of Steric Structure of Catalytic Protein for Nitrile Synthesis – Contribution to development of environmentally friendly nitrile production method using aldoxime dehydratase - (Press Release)
 ,
, .
.