Why Is the Toxicity of Thermostable Direct Hemolysin (TDH) Produced by Vibrio parahaemolyticus, a Bacterium Causing Food Poisoning, Not Eliminated by Heating? – Structural Clarification of TDH - (Topic)
- Release Date
- 21 May, 2010
- BL41XU (Structural Biology I)
- BL40B2 (Structural Biology II)
Osaka Medical Center and Research Institute for Maternal and Child Health
Research Institute for Microbial Diseases, Osaka University
Yokohama City University
|
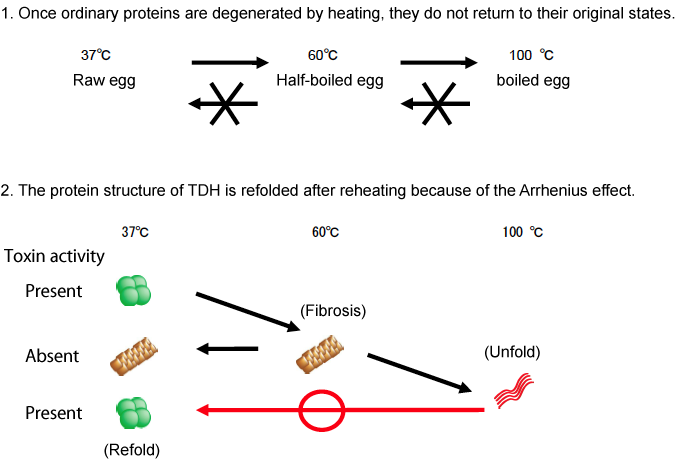
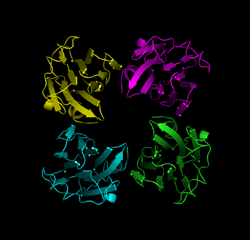
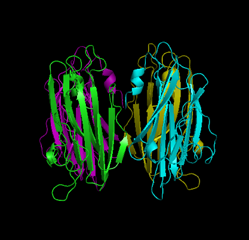
Vibrio parahaemolyticus was first discovered by the late Tsunesaburo Fujino (professor emeritus of the Research Institute for Microbial Diseases, Osaka University). It was the bacterium responsible for the large-scale food poisoning due to the consumption of contaminated boiled and dried sardine fries (Shirasu-boshi) that occurred in the south of Osaka Prefecture in October 1950 affecting 272 people and killing 20 of them. Most of the sources of food poisoning caused by V. parahaemolyticus are fish and shellfish. V. parahaemolyticus is still one of the major causative bacteria in cases of food poisoning that often occur in summer. Thermostable direct hemolysin (TDH), a protein toxin produced by V. parahaemolyticus, has been reported to show intestinal toxicity, cardiotoxicity, hemolytic activity, and cytolethal activity. The term thermostable, as in the name of TDH, indicates a unique property of the toxin that, although it is deactivated by heating to 60°C, the toxin activity is reactivated after the toxin is heated to higher temperatures (i. e., the Arrhenius effect, which was first discovered in the α toxin of Staphylococcus aureus by the Nobel Prize winning scientist Svante Arrhenius in 1907). Generally, food poisoning bacteria are killed by heating. However, the toxin activity of TDH is known for its intractable property of being reactivated by heating at high temperatures. A joint research group consisting of Itaru Yanagihara, a head of department of the Osaka Medical Center and Research Institute for Maternal and Child Health, and Takeshi Honda, a professor emeritus of the Research Institute for Microbial Diseases, Osaka University, and their colleagues, has led the world in clarifying the Arrhenius effect. They have already discovered that TDH assembles into fibrous aggregates (fibrosis) when heated to 60°C, but that the deactivated fibrous TDH is unfolded by further heating (the protein is loosened) and then refolded (rewound) under optimum conditions (Fig. 1). In this study, through a joint research with Hiroshi Hashimoto, an assistant professor of Yokohama City University, the three-dimensional structure of TDH was successfully clarified at atomic-level resolution (Fig. 2) at synchrotron radiation facilities [Structural Biology I Beamline BL41XU at SPring-8 and NW12A Beamline at the High Energy Accelerator Research Organization (KEK)]. It has long been known that TDH is a protein toxin that forms a pore on the cell membrane. In the present analysis, a pore with a diameter of approximately 2 nm and a depth of approximately 5 nm was observed at the center of the TDH tetramer. The pore was also observed in the single-particle analysis of the protein under an electron microscope and in X-ray solution scattering analysis using the Structural Biology II Beamline BL40B2 at SPring-8. Moreover, in the molecular simulation using a supercomputer, more than 600 water molecules were observed to pass through the pores of TDH in the solution in only 10 ns. The results of the structural analysis of TDH were published in the scientific journal, Journal of Biological Chemistry (JBC), on 21 May 2010. The toxin structure of the bacterium causing food poisoning that often occurs in summer because of the Japanese seasonal food culture was clarified in the analysis using the world's largest synchrotron radiation facility and a supercomputer. Cutting-edge technological development of these facilities and their maintenance are essential for academic progress and the improvement of public health in Japan. Publication: |
<Figure>
For more information, please contact: Dr. Hiroshi HASHIMOTO (Yokohama City University) |
- Current article
- Why Is the Toxicity of Thermostable Direct Hemolysin (TDH) Produced by Vibrio parahaemolyticus, a Bacterium Causing Food Poisoning, Not Eliminated by Heating? – Structural Clarification of TDH - (Topic)