Elucidating the Mechanism behind Light-induced Water-splitting in Photosynthesis (Press Release)
- Release Date
- 18 Apr, 2011
- BL44XU
- BL41XU
- BL38B1
Okayama University
Osaka City University
|
Scientists of a research group led by Professor Jian-Ren Shen (Division of Bioscience, Graduate School of Natural Science and Technology, Okayama University) and Professor Nobuo Kamiya (The OCU Advanced Research Institute for Natural Science and Technology, Osaka City University) have elucidated the mechanism behind the splitting of water and the evolution of oxygen during photosynthesis by using solar energy. This will lead to the clarification of the mechanism by which solar energy is converted to chemical energy that living organisms can use, and is a groundbreaking achievement that can contribute to solving problems related to the global environment, energy, and food shortages. The results of this study were published online as a research article in the British scientific journal Nature on 17 April 2011. Publication: |
Outline
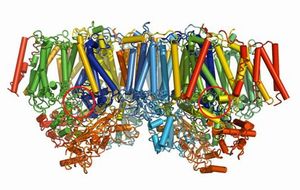
In photosynthesis, glucose is generated from carbon dioxide, a combustion residue of organic substances, using solar energy. Glucose is a nutritional source, from which energy is obtained by almost all living organisms on the earth through respiration. Upon the reception of solar light, photosystem II (PSII, Fig. 1) splits water to evolve oxygen molecules and electrons simultaneously. These electrons are used to convert carbon dioxide to glucose. Traditionally, the evolution of oxygen by PSII was considered to progress on a metal-oxygen cluster, in which four manganese (Mn) atoms and a calcium (Ca) atom are linked by multiple oxygen (O) atoms. However, the accurate chemical composition and detailed atomic arrangement of the cluster remained unclear.
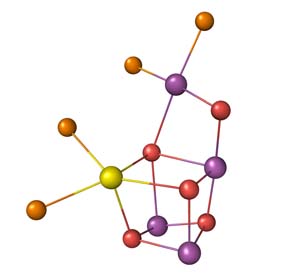
In this study, the scientists succeeded in markedly improving the quality of PSII crystals and carried out X-ray crystal structure analysis using three beamlines (BL44XU, BL41XU, and BL38B1) at SPring-8. It was found that the cluster has the chemical formula of Mn4CaO5 and has the distorted chair shape as its overall structure, in which two water molecules are bound to each Mn and Ca atom (Fig. 2). Any of these four water molecules are considered to be incorporated into the oxygen molecules evolved from the Mn4CaO5 cluster.
Benefits expected
If catalysts that imitate the structure of the above cluster can be developed in the future, it will become possible to combine the transmission of solar energy to catalysts and the synthesis of hydrogen molecules and methanol using electrons generated from water by the catalysts, to realize artificial photosynthesis. This is expected to contribute to solving problems related to energy, the environment, and food shortage that we will face in the near future.
<<Figures>>
PSII has a dimer structure consisting of two monomers. The oxygen-evolving centers are positioned at the sites indicated by two red circles.
The purple spheres represent Mn atoms, the yellow sphere represents a Ca atom, the red spheres represent O atoms that link the metal atoms, and the orange spheres represent O atoms of water molecules that are involved in the evolution of oxygen.
|
For more information, please contact: Nobuo Kamiya (Osaka City University) |
- Previous Article
- Establishment of Method for Developing Stratum Corneum in Three-Dimensional Cultured Skin Models- Realized using SPring-8 beamline(Press Release)
- Current article
- Elucidating the Mechanism behind Light-induced Water-splitting in Photosynthesis (Press Release)