Preparation of Rock-Salt-Type Crystal of Li+-Encapsulated Fullerene C60 and Clarification of Its Structure (Press Release)
- Release Date
- 29 Feb, 2012
- BL02B1 (Single Crystal Structure Analysis)
- BL02B2 (Powder Diffraction)
Nagoya City University
Nagoya University
Tohoku University
Idea International Corporation
Scientists of Nagoya City University have discovered that, when encapsulating a lithium cation (Li+), spherical carbon molecule fullerenes (C60)*1 pair with specific anions such as hexafluorophosphate (PF6-) ions to form a rock salt (NaCl)*2-type crystal. This was discovered jointly with scientists of Nagoya University, Tohoku University, and Idea International Corporation. The detailed analysis of this crystal structure using the facilities of SPring-8*3 revealed that Li+ is localized at certain positions below -200 oC, although it freely moves within C60 at room temperature. It was also clarified that Li+-encapsulated C60 (Li+@C60) thermally expands with decreasing temperature (negative thermal expansion*4). These results led to the following findings: the electrostatic properties of Li+@C60 are similar to those of metal cations such as sodium ions (Na+) and the position and movement of Li+ encapsulated in C60 strongly depend on the environment around the molecule such as the temperature and the arrangement of anions. These characteristics of Li+@C60 may be applied to nanosized electronic devices that operate at the level of single molecules. The achievements of this study were obtained mainly by the following scientists: Shinobu Aoyagi (Associate Professor) of Nagoya City University; Ryo Kitaura (Associate Professor), Eiji Nishibori (Associate Professor), Hisanori Shinohara (Professor), and Hiroshi Sawa (Professor) of Nagoya University; Hiroshi Okada (Assistant Professor) and Hiromi Tobita (Professor) of Tohoku University; and Dr. Yasuhiko Kasama of Idea International Co., Ltd. The results were published online in the German chemistry journal Angewandte Chemie International Edition on 28 February 2012 prior to publication in the printed version as a hot paper, i.e., a highly noteworthy paper, on 2 April. Publication: |
<<Figures>>
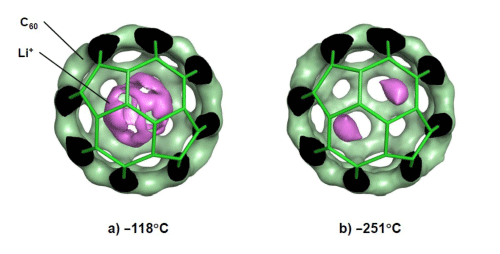
Li+@C60 and PF6- are paired to form a rock-salt-type crystal structure.
The purple regions represent positions where Li+ exists (i.e., the electron distribution), the green regions represent the electron distribution of C60, and the green bars represent the framework of C60. a) Li+ appears to spin around its position while slightly deviating from the center of C60 at -118 oC, whereas b) Li+ mainly exists at two polar positions with an equal probability at -251 oC because of its reduced movement.
<<Glossary>>
*1 Fullerene (C60)
C60 is a hollow spherical molecule that consists of 60 carbon (C) atoms with an arrangement giving it the appearance of a soccer ball. The existence of C60 was predicted by Eiji Osawa in 1970, and it was identified in 1985 by Harold Kroto and colleagues, for which they were awarded the 1996 Nobel Prize in Chemistry. C60 can now be mass-produced and is used in various products such as sports equipment, cosmetics, and solar cells. Metal atoms and gas and water molecules can be encapsulated in the hollow interior of C60; in particular, fullerenes encapsulating metal atoms are called metal-encapsulated fullerenes.
*2 Rock salt (NaCl)
Rock salt is the mineralogical name for sodium chloride (NaCl), the principal component of salt. In the NaCl crystal, sodium ions (Na+) and chloride ions (Cl-) are paired and alternately arranged in a regular fashion, which is known as the rock-salt-type crystal structure (Fig. 1a). Rock salt is the most well-known example of an ionic crystal.
*3 SPring-8
SPring-8 is a facility that generates the world's highest-performance synchrotron radiation, which is available for public use. It is located in Harima Science Garden City in Hyogo prefecture. Synchrotron radiation refers to the intense electromagnetic waves (X-rays) that are generated when the traveling direction of electrons accelerated to near the speed of light is bent by electromagnets. The name SPring-8 is derived from Super Photon ring-8 GeV.
*4 Negative thermal expansion
Negative thermal expansion refers to the phenomenon of the volume of a substance decreasing with increasing temperature. For most substances, their volume increases with increasing temperature and decreases with decreasing temperature. This is referred to as thermal expansion, for which the rate of change in volume with respect to temperature (i.e., the coefficient of thermal expansion) is positive. In contrast, the volume of some substances decreases with increasing temperature and increases with decreasing temperature. This is called negative thermal expansion because the coefficient of thermal expansion is negative.
|
For more information‚ please contact: |
- Previous Article
- Discovery of New Structural Change of Polymer Melt (Press Release)
- Current article
- Preparation of Rock-Salt-Type Crystal of Li+-Encapsulated Fullerene C60 and Clarification of Its Structure (Press Release)
](/en/news_publications/press_release/2012/120228_2_fig/fig1.png)